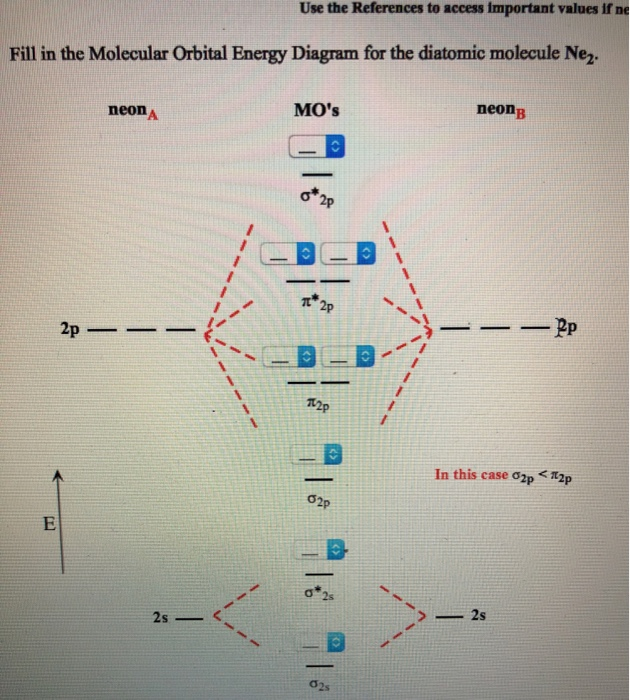
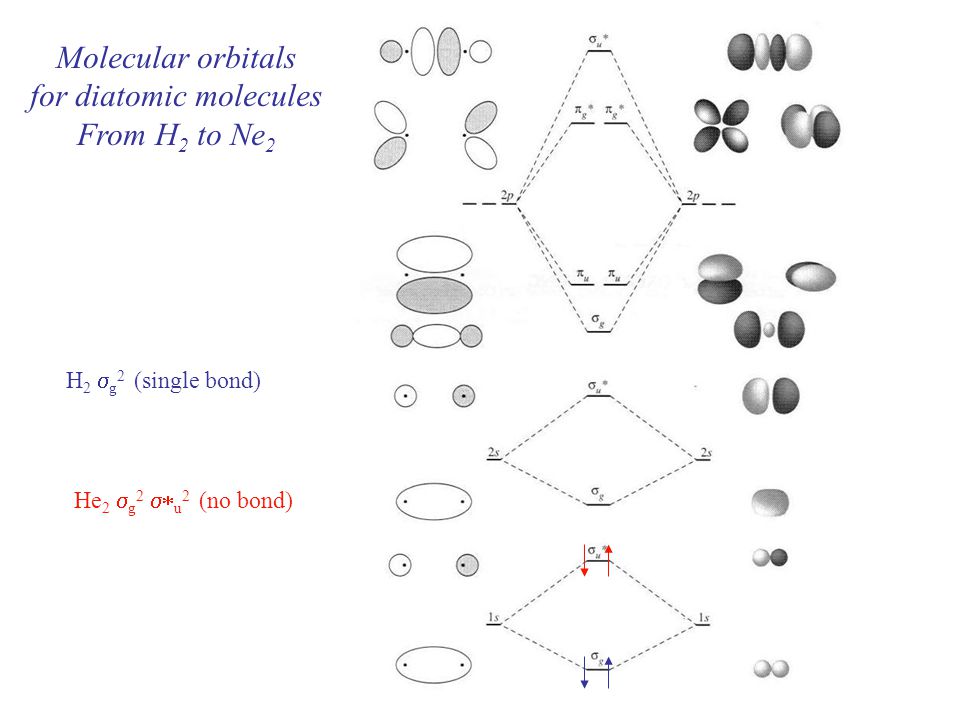
Molecular Orbital Diagram Ne2
Use molecular orbital theory to predict molecular geometry for simple triatomic systems • rationalize molecular structure for several specific systems in terms based on energy, which can interact?
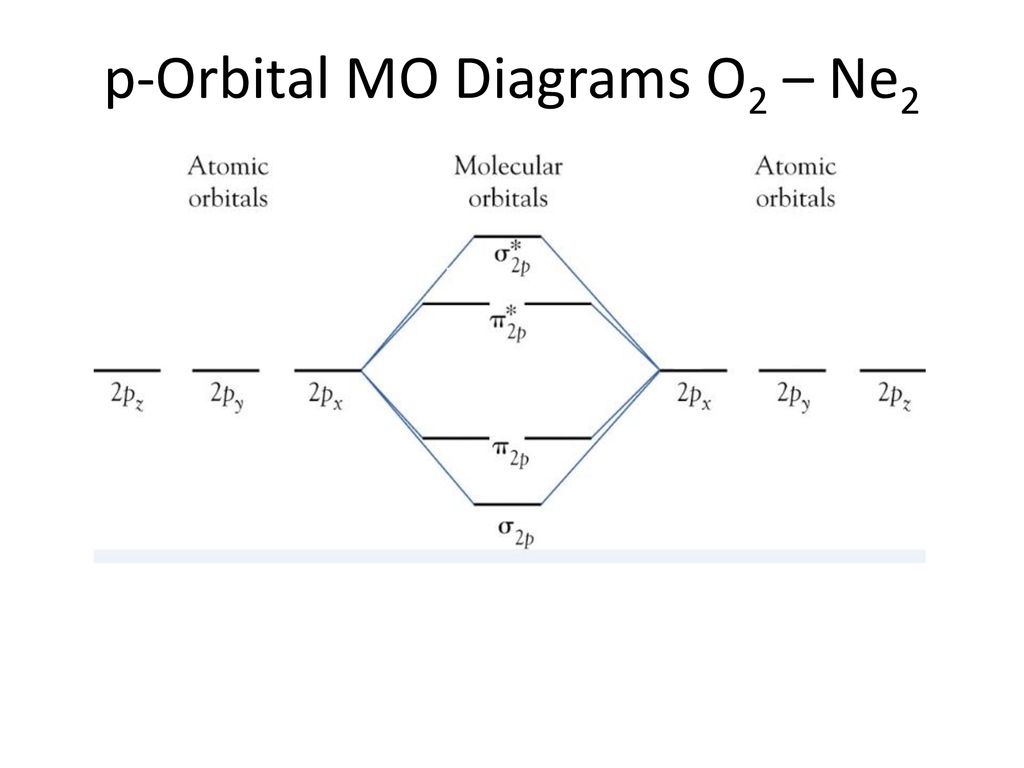
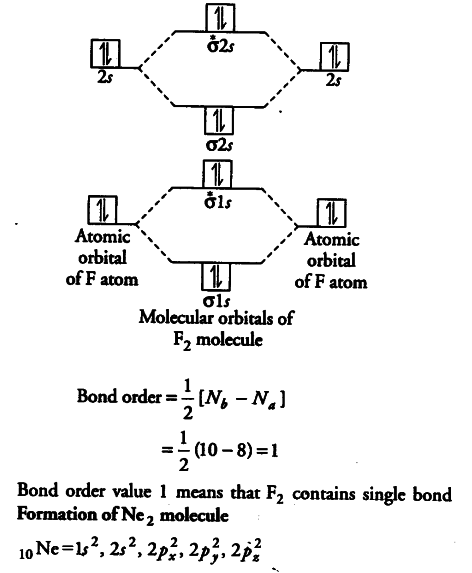
Molecular orbital diagram ne2. If ne 2 did form, it would be diamagnetic. Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the pauli principle, the what is the molecular orbital diagram for the diatomic neon molecule, ne2? Let me explain the molecular orbital diagram of n2 using its diagram. Now note that even in this advanced molecular orbital theory a bunch of approximations is introduced, and the answer in general depends on at which level of theory calculations are done.
The molecular orbital diagram of hypothetical molecule is given in the attachment. Among those elements there are some traits that are always true Fill from the bottom up, with 9 valence electrons total. The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those that construct a molecular orbital diagram of the kind shown in this lesson for a simple diatomic molecule, and indicate.
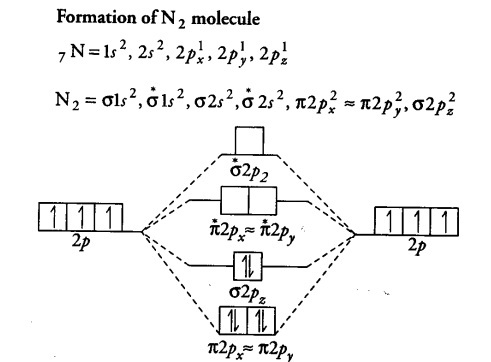
| online chemistry tutorial iit, cbse chemistry, icse chemistry, engineering and molecular orbital diagram of c2 molecule : One is for the elements up to nitrogen. One atom of nitrogen has 7 electrons so a n2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bon. The orbital correlation diagram in predicts the sa.
2p 2s mo diagram for n2 n2, o2, f2, ne2? Molecular orbital diagram for nitrogen gas ( 1 ion) (n2( )). The mos for the valence orbitals of the second period are shown in figure 12. The molecular orbital diagram shows the creation and filling of mos in a bond.
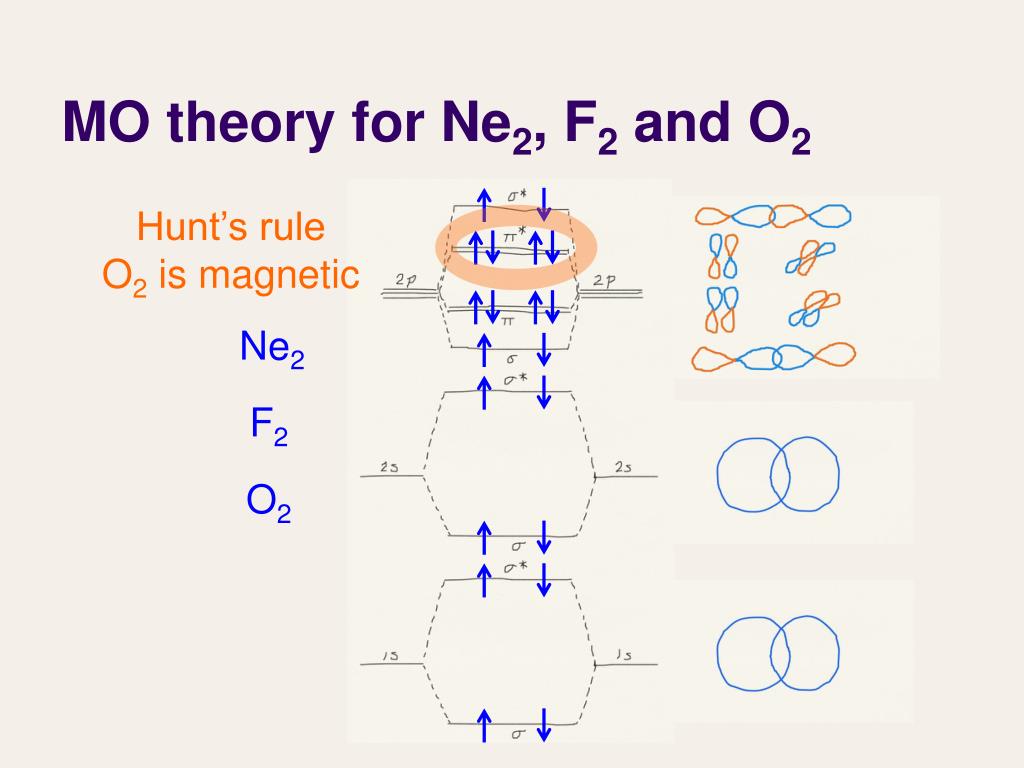
As it can be seen from the mot of o2 , the electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Unlike most texts on molecular orbital theory and quantum mechanics, this text treats polyatomic molecules before linear molecules before atoms. B, c, n, o, f, ne. Be sure your diagram contains all of the electrons in the ion, including any core electrons.
The molecular orbital diagram of are shown below. The video below describes how to generate molecular orbital diagrams for b₂ and other diatomic molecules from row 2 elements of the. Neon atom has 10 electrons and its electronic configuration is. Number of electrons in c2 molecule = 12.
Each line in the molecular orbital diagram represents a molecular orbital, which is the volume within which a high percentage of the negative charge generated by the for o2, f2, or ne2, the orbital energies are different enough so only orbitals of the same energy interact to a significant degree. 168 chapter 8 & 9 covalent bonding and molecular structures we can describe diatomic molecules the molecular orbital diagram predicts co to be very stable with a bond order of three. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2). (b) the shapes of the molecular orbitals are obtained by squaring the.
The other is for after nitrogen. Heteronuclear diatomic molecules how do heteronuclear mos. The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. Individual atomic orbitals ao are arranged on the far left and far right of the diagram.
Looking at ne2 molecular orbitals, we see that the order is consistent with the generic diagram. The molecular orbital energy diagram predicts that he2 will not be a stable molecule, since it has equal numbers of bonding and antibonding however, we can predict that the be2 molecule and the ne2 molecule would not be stable. 1s 1s 2s 2p 2p 2s 2p 2p 2p 2p we predict the nitrogen. Each molecular orbital can only have 2 electrons, each with an opposite spin.
The bond order of is, 3. Will the mo diagram be the same as that of $\ce{n2}$ or not? There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). A molecule in which all the electrons are paired, is called diamagnetic.
Valence on the other hand, molecular orbital theory visions the electrons of a covalent bond to be delocalized over the entire molecule. The molecular orbital (mo) theory is a way of looking at the structure of a molecule by using molecular orbitals that belong to the look now at the mo diagram for oxygen, which is shown in figure 4. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2). Drawing molecular orbital diagrams is one of the trickier concepts in chemistry.
The first major step is understanding the difference between two major theories: • because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. We can see this by a consideration of the molecular electron. According to the molecular orbital theory, the general molecular orbital configuration will be, as there are 7 electrons present in nitrogen.
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Within the diagram, orbitals are represented by horizontal lines. Be sure your diagram contains all of the electrons in the ion, including any core electrons.