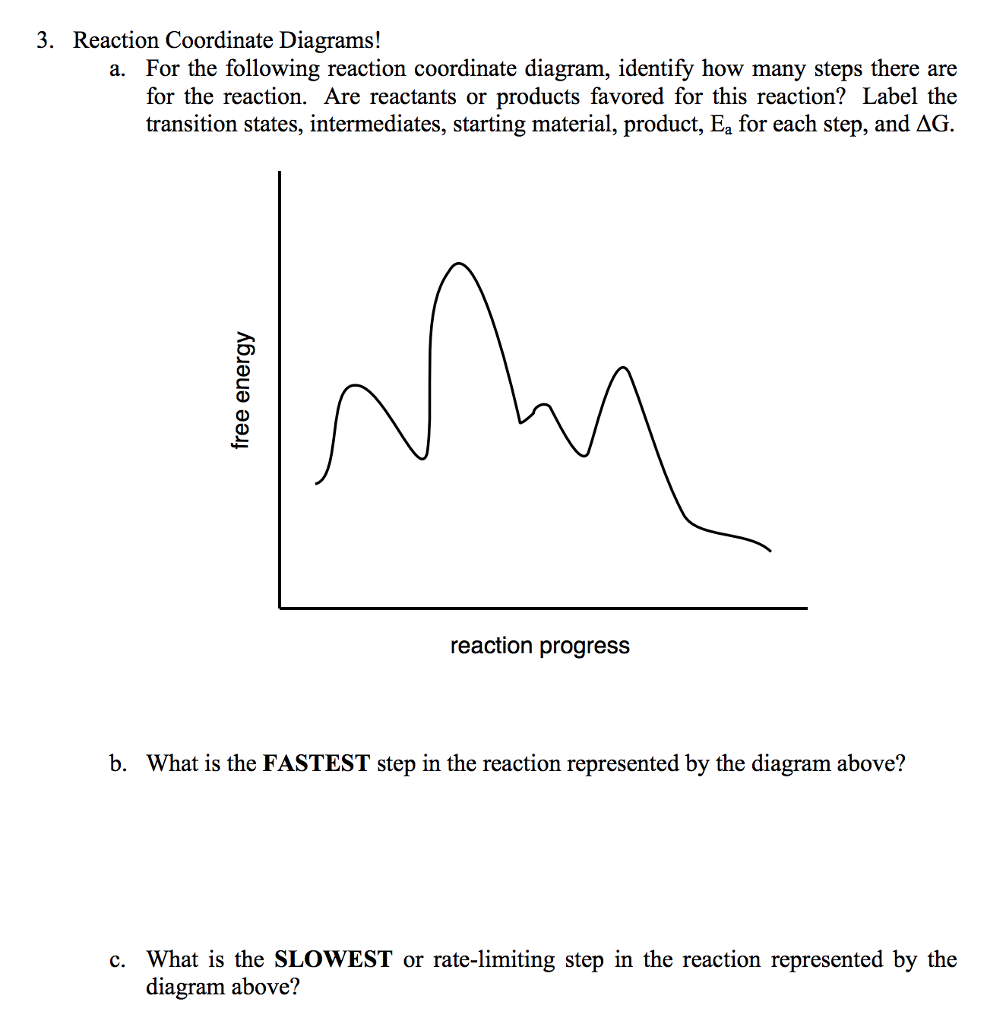
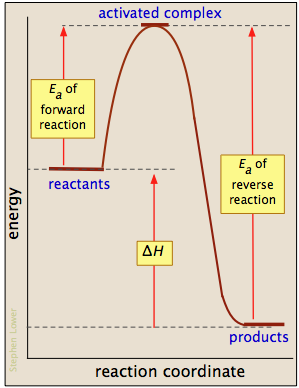
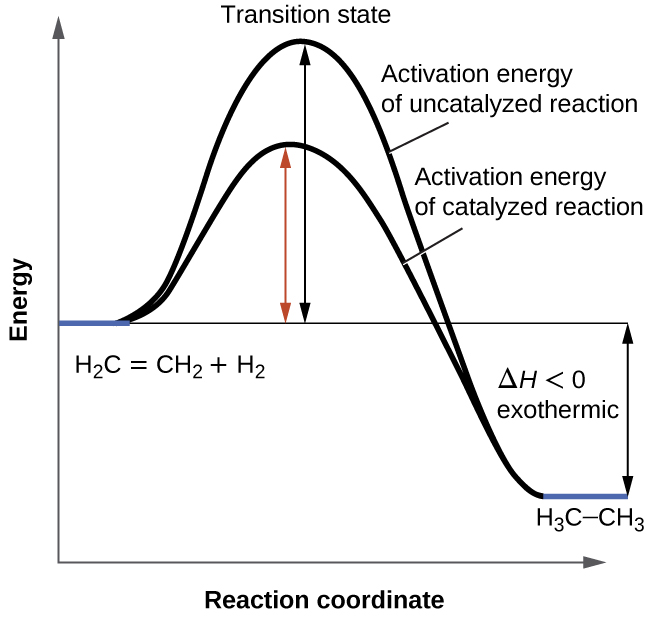
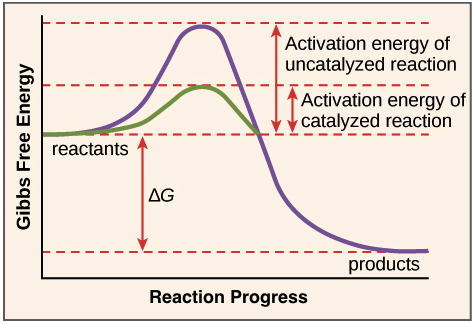
Label The Following Reaction Coordinate Diagram
Let's say you're looking at a reaction coordinate diagram for an exothermic reaction.
Label the following reaction coordinate diagram. Draw the major organic product(s) for the following reaction. Energy reactant(s) transition state product(s) activation energy (forward) energy enthalpy of enthalpy of reaction product(s) reaction ahrxn reactant(s) reaction coordinate reaction coordinate reset. The function, which depends on magnitude of the rate constants describing individual steps in the reaction, reaches a. Transcribed image text from this question.
Note the use of a generic base in the last step. This reaction exothermic or endothermic ? .reaction coordinate diagram) the transition state(s) (2 points). I am trying to find a transition state using qst2 with b3lyp, but i am getting the following error new curvilinear step not converged.
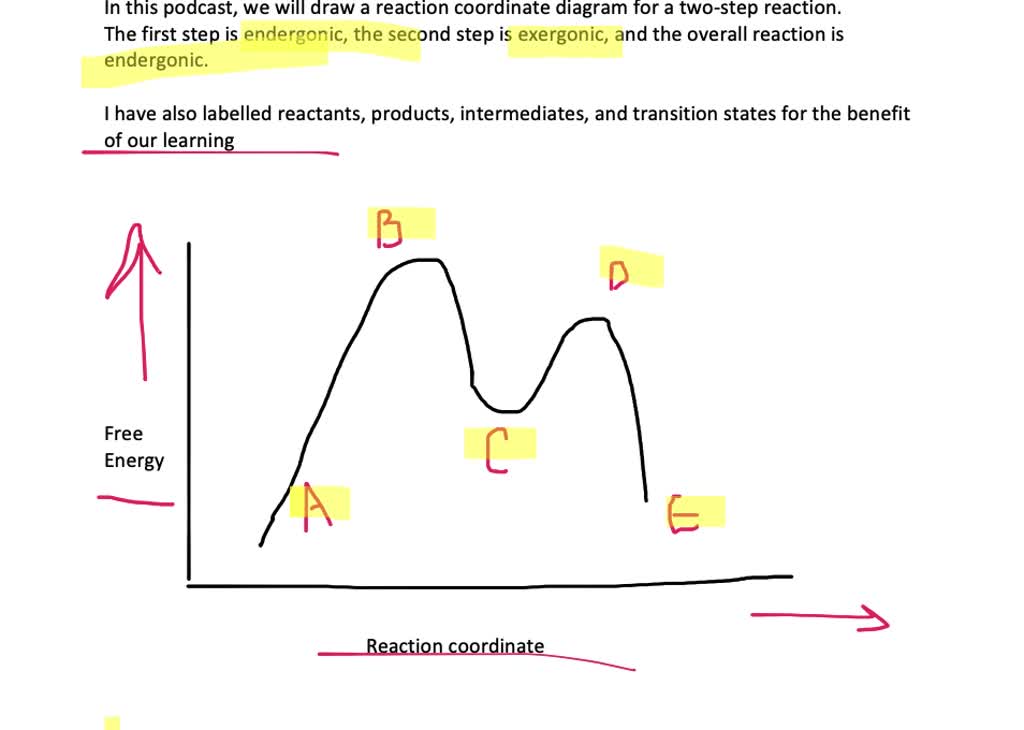
I labelled all necessary details for your convenient. Label the following reaction coordinate diagram. The energy of the activated complex c. Label (on the reaction coordinate diagram) the intermediate(s) (2 points).
Use the following search parameters to narrow your results umm, draw a diagram in latex or gnuplot or both. The following 19 files are in this category, out of 19 total. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. (a) how many elementary steps are in the examine the reaction coordinate diagram given here.
(circle one) heat is released as a product the entropy (randomness) of the system increasing or decreasing ? For the following reaction 7. The reaction has two transition states, the first transition state. ●colour of salt changes because it comes in contact with sunlight.
Energy diagrams the hammond postulate for each of the diagrams below, will the transition state structure nucleophiles and electrophiles reactgeneral organic reactions • draw all possible resonance structures for the following molecule. I don't think there are any fancy ways of doing this, as many articles have crappy reaction coordinate diagrams. Show a free energy versus reaction progress diagram for the following reaction: (3 points) draw a reaction coordinate diagram for a simple endothermic reaction, label your axes, and on your figure clearly label.
Media in category reaction coordinate diagrams. Make sure you keep the diagram proportional to your data. The potential is given by the following expression,38. Enter relative energies (in kcal/mol) of all minima and maxima on the potential energy surface from the command line.
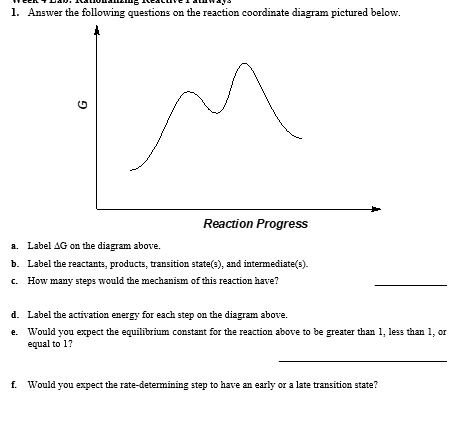
B) label the reaction coordinate diagram for a typical eas reaction shown below by correctly placing. Observe carefully andanswer the following questionscho… ●the decomposition reaction that takes place in the presence of sunlight is called photo decomposition reaction. Example, which of the following two reactions a or b is faster?? Label the reactants, products, intermediates, and transition.
Be sure you have really obtained ts, that means stationary point, i.e following is an example of reaction coordinate diagram. A typical reaction coordinate diagram for a mechanism with a single step is shown below there will also be n maxima representing the n transition states. A simple script to draw a reaction energy diagram using bezier curves. Intrinsic reaction coordinate calculation— both sides of ts lead to same minimum.
From wikimedia commons, the free media repository. Indicate if reactants, products, or neither are favored for the reaction by circling the correct. Please note that the blue path is for the. A quantitative expression developed by albery and knowles to describe the effectiveness of a catalyst in accelerating a chemical reaction.
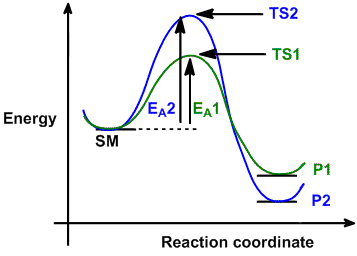
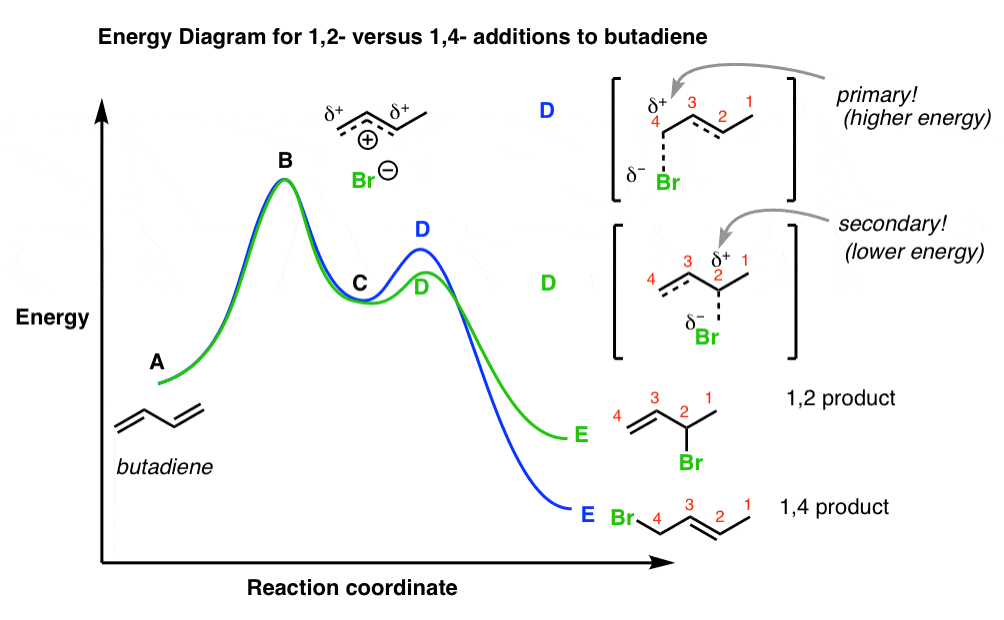
Which is the fastest reaction?formation of c from aвformation of e from cformation of a from cdo formation of c from e3. For example, a reaction with three elementary steps could have the following reaction coordinate diagram. Despite its apparent simplicity, this energy diagram conveys some very important ideas about the thermodynamics and kinetics of the reaction. Since the an sn1 mechanism consists of two elementary steps, there are two transition states in the reaction coordinate diagram.
Potential energy of the products? Yet, if we have two reactions with activation energies #e_a# and #e_a'# and reaction energies #deltae# and #deltae'#, then we should have #|deltae_(fwd). Label (on don't forget about formal charges if applicable! Consider the following reaction energy diagram:
.the correct reaction coordinate diagram and draw the major organic product.which of the following reaction coordinate diagrams corresponds to the reaction?draw product b. Review and cite intrinsic reaction coordinate protocol, troubleshooting and other. As they are, the values don't seem to make sense. There is an #e_a' (fwd)# and an #e_a' (rev)#, which seem to indicate the same reaction.
Which of the following reaction coordinate diagrams is the only one that can represent a reaction that occurs via a carbocation? If dg depends on the. • the diagram shows the energy is of the entire system (not just the alkene and fragments) as a function of progression along the reaction coordinate. Activation energy of the forwird.