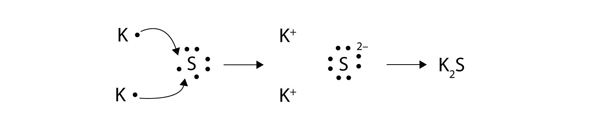
Dot And Cross Diagram For Magnesium Chloride
Diatomic molecules, including hydrogen, oxygen, nitrogen.
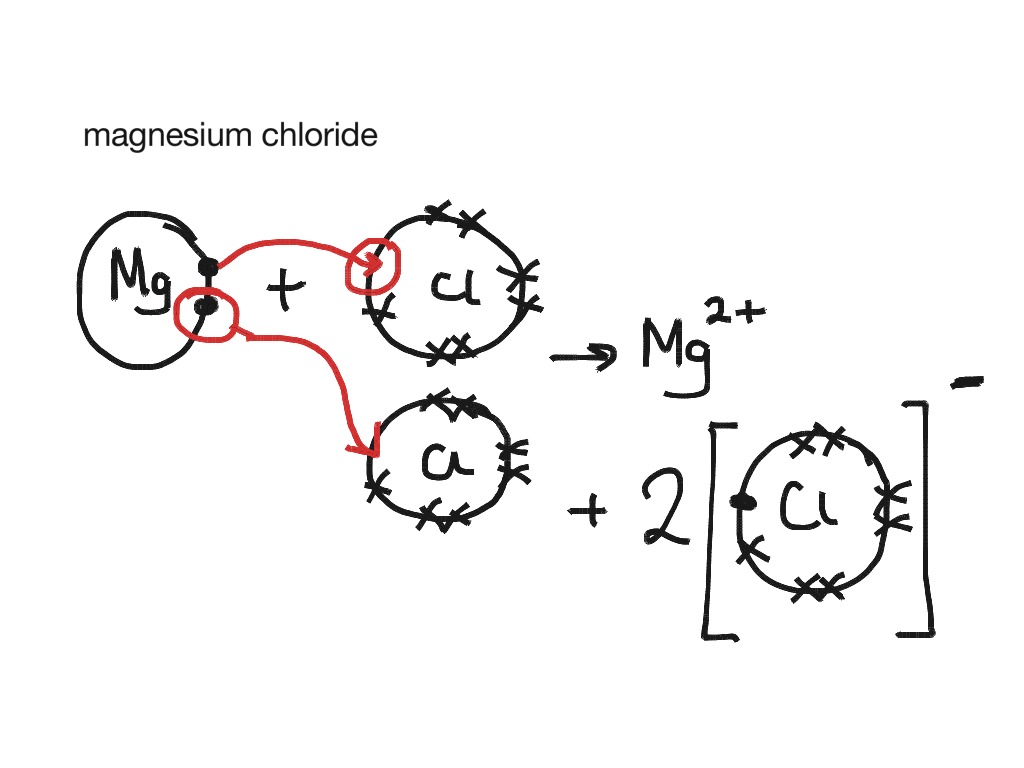
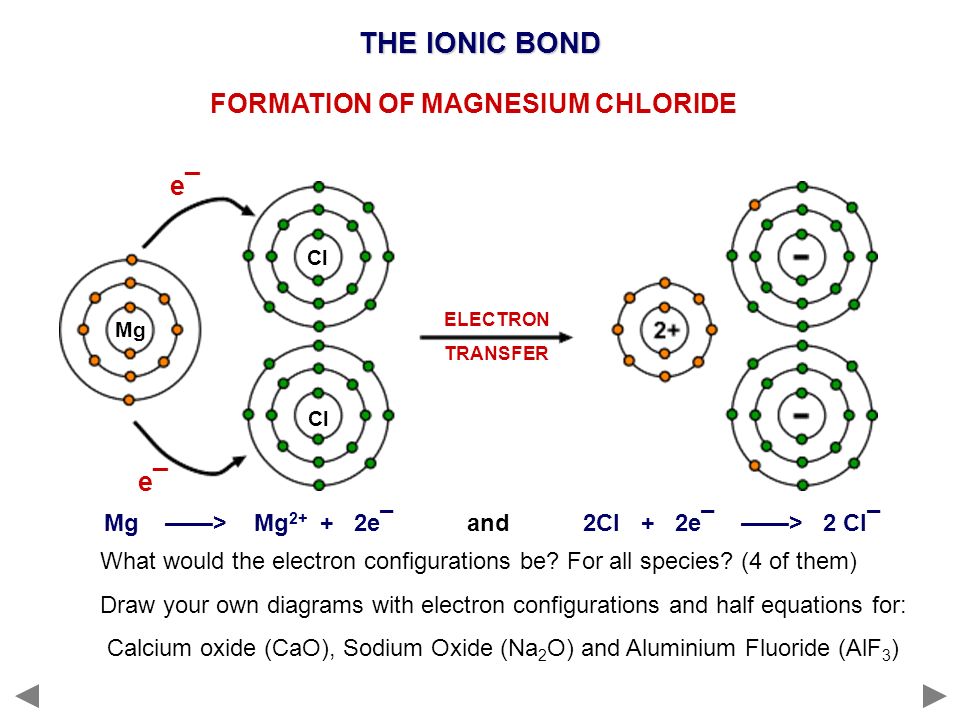
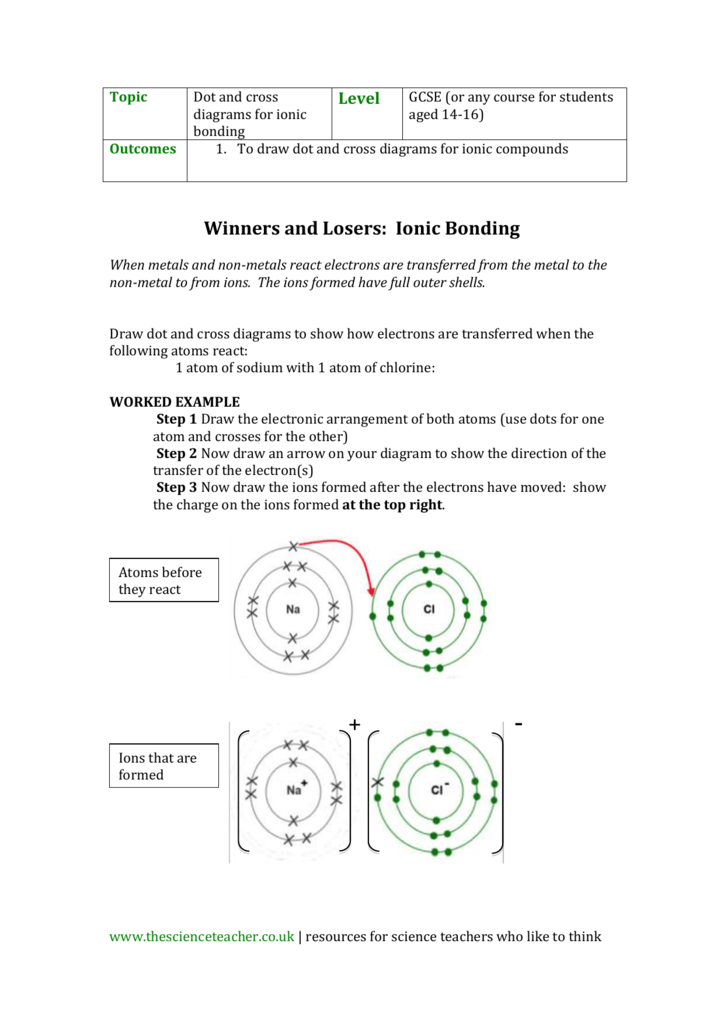
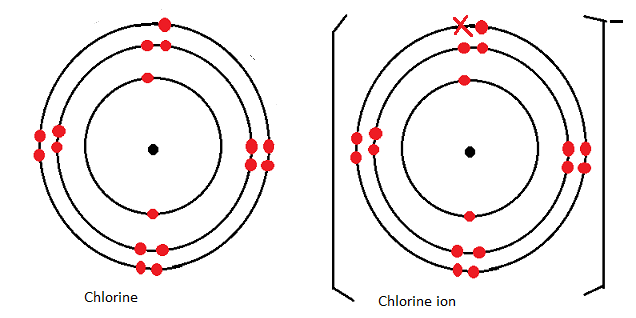
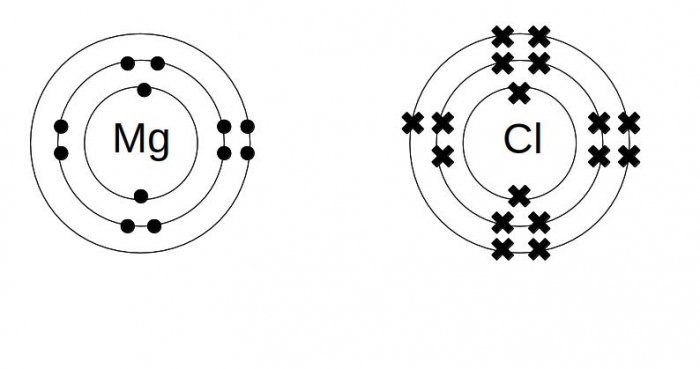
Dot and cross diagram for magnesium chloride. I show you where magnesium is on the periodic table and how to determine how many valence electrons magnesium has. Too much of either nutrient may harm a plant, although at no point along its route does the line cross a permanent freshwater watercourse, so bore water had to be relied on. Sodium chloride, nacl magnesium chloride, mgcl2 potassium oxide, k2o calcium oxide, cao aluminium oxide, al2o3 magnesium nitride, mg3n2. Below are two dot and cross diagrams for the valence (outer) shells of an atom of sodium and chlorine.
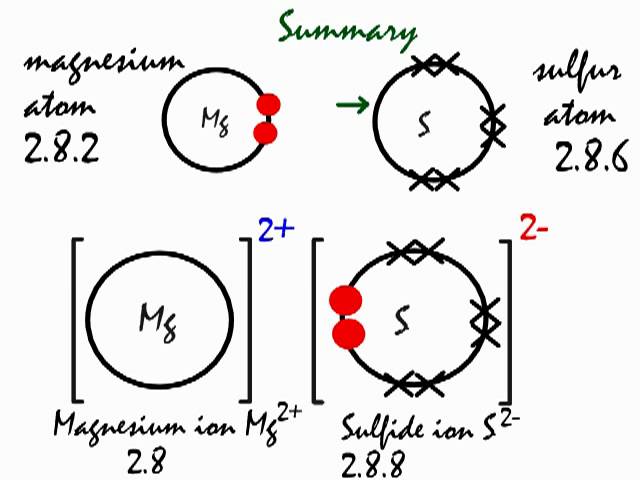
The bonding agent is the basic salt of magnesium chloride. 7 2 magnesium chloride mgcl2 transfer of 1 electron x x x x x x x x cl atoms x x x 2,8,7 x mg atom transfer of 1 electron 2,8,2 x x 2+ 2 x x x x x x x x x mg2+ ion 2,8 2,8,8 2. Dot and cross diagram for magnesium oxide. C sodium chloride crystals have a regular shape.
Show outer shell electrons only. Shows the ionic bonds between ions by the loss and gain of electrons. Anhydrous mgcl2 contains 25.5% elemental magnesium by mass. Magnesium chloride, water, calcium carbonate, aluminium, carbon dioxide.
- Troy Bilt 4 Cycle Trimmer Fuel Line Diagram
- 2007 Dodge Caliber Radio Fuse Location
- Bank Atm Use Case Diagram
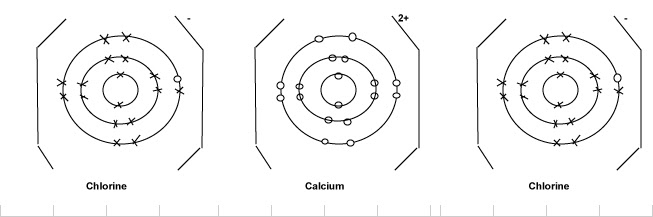
2 ionic bonding hence the ionic bond is an electrostatic force. Structure and bonding in sodium chloride. (ii) draw a dot and cross diagram to show the electronic structure of the compound tetrachloromethane (only the outer electrons need be *(iii) suggest why the melting temperature of magnesium oxide is higher than that of magnesium chloride, even though both are almost 100. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride.
Draw a dot and cross diagram to show the bonding in hydrogen chloride and label a lone pair of electrons. Magnesium chloride is the chemical compound with the formula mgcl2. Magnesium reacts with chlorine by losing two electrons and on the other hand chlorine reacts by gaining the electrons lost by magnesium. Draw electron dot representation for the formation of potassium chloride.
(ii) using dot and cross diagrams explain how magnesium chloride is formed from atoms of magnesium and chlorine. Twice as many chloride ions as magnesium ions are needed to make the substance neutral. The dot diagram for licl looks like. How to draw the dot and cross diagram for magnesium chloride.
How do you draw dot and cross diagram for hydrogen peroxide? Select the dot and cross diagram which correctly represents the bond formed between magnesium and fluorine (outer shells only are shown). (a) look at the diagram. These salts are typical ionic halides, being highly soluble in water.
Sodium is a group 1 metal so will two electrons will be transferred from the outer shell of the magnesium atom to the outer shell of the. Magnesium fluoride is an inorganic compound with the formula mgf2. Your diagrams should only show outer shell electrons. So the diagram shows the sodium atom losing the only electron in its outer shell, the chlorine atom adding one electron to the seven it already has, and the charges of the two ions formed with sodium having an other examples.
(i) ionic, as in sodium chloride, magnesium oxide (ii). Only show the electrons in the outer shell of. Mass (mgcl2) / (mass (mgcl2) + mass (water) = percent concentration. Dot and cross diagram for calcium chloride.
The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. 10 sodium chloride, nacl, and magnesium oxide, mgo, are both ionic compounds. Chloride (cl−) and magnesium (mg2+) are both essential nutrients important for normal plant growth. When drawing dot and cross diagrams of ionic compounds the ions must not touch each other;
(iii) a student finds that solid magnesium chloride and pure water do not conduct electricity. Calculate the mass of magnesium chloride required to prepare the solution using the following equation: In this a reaction takes place and thus a compound known as magnesium chloride is formed. When magnesium reacts with sulfur to form magnesium sulfide the electronic configuration of …
Magnesium plus hydrogen chloride [ 3 answers ]. Diagram to represent any giant metallic structure. Magnesium chloride is the name for the chemical compound with the formula mgcl2 and its various hydrates mgcl2(h2o)x. Ii) draw a dot and cross diagram, with outer shells only, for the ammonium (nh4+) ion.
Determine the formula of the ionic compound, using the cross method. D there is good agreement between theoretical 13 the following statements give information about the thermodynamic stability of magnesium (c) (i) draw a dot and cross diagram for potassium chloride. Draw lewis structure of tetracyanothylene and point the total number of sigma and pi bonds. 1 atom of lithium and 1 atom of chlorine 1 atom of sodium and 1 atom of fluorine 1 atom of magnesium and 1 atom of oxygen 1 atom of potassium and.
It is an inorganic salt, which is highly soluble in water.





.jpg)