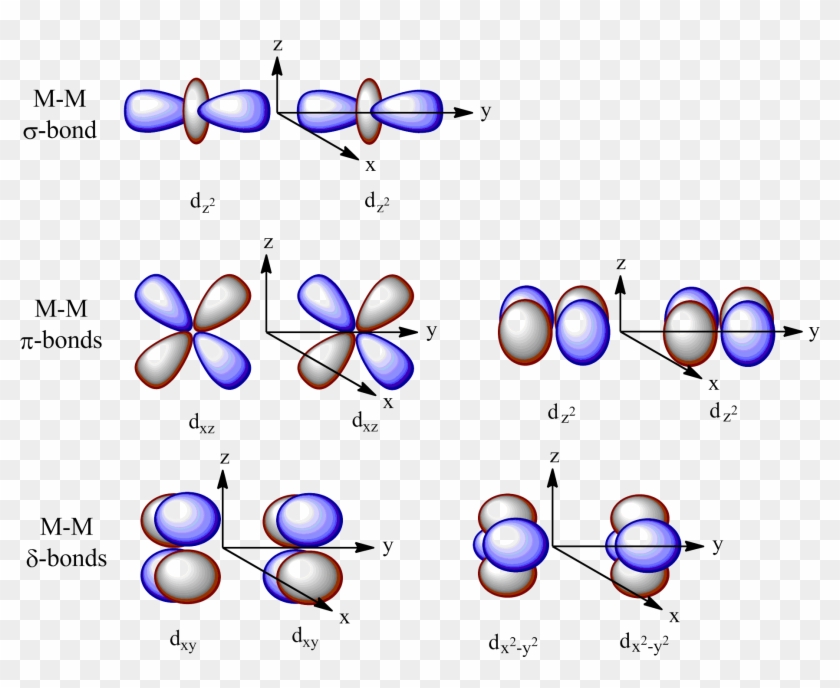
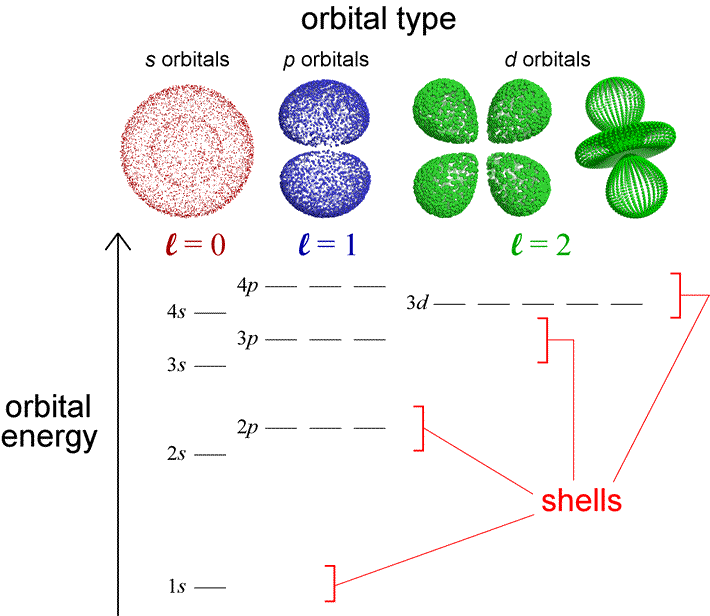
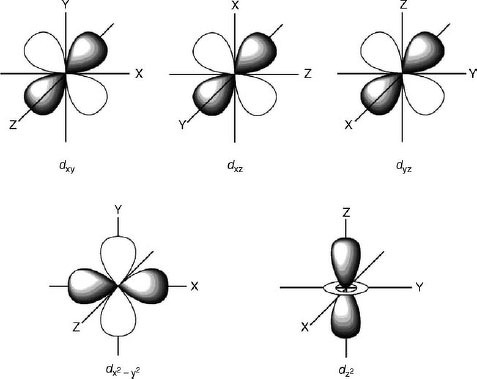
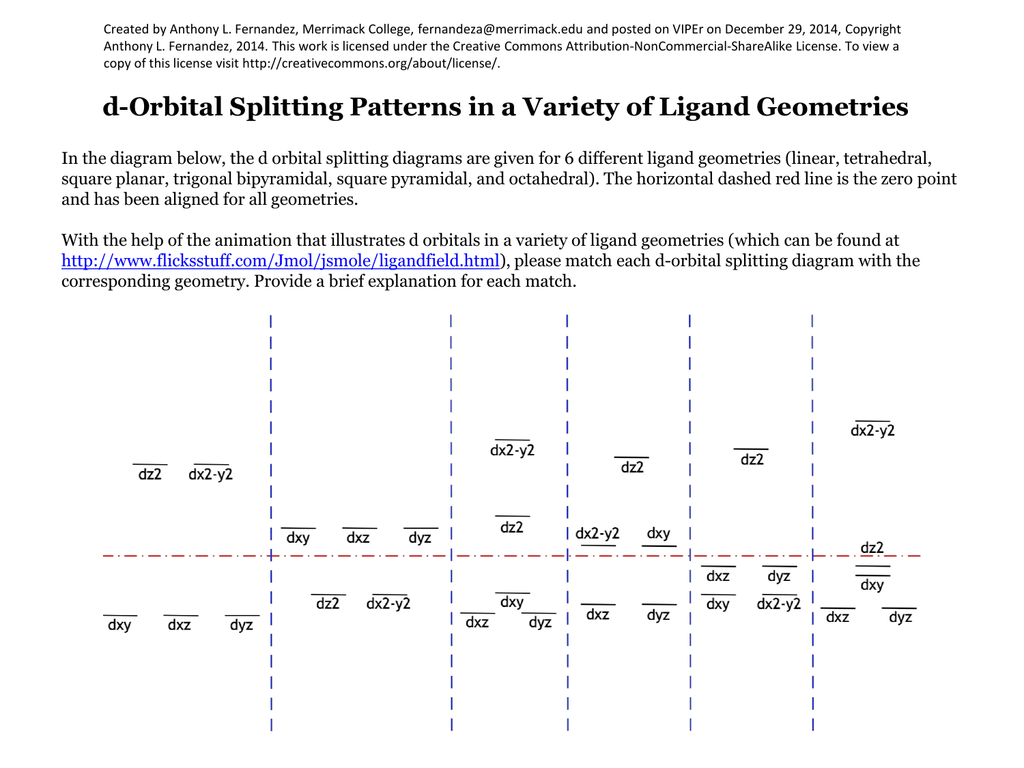
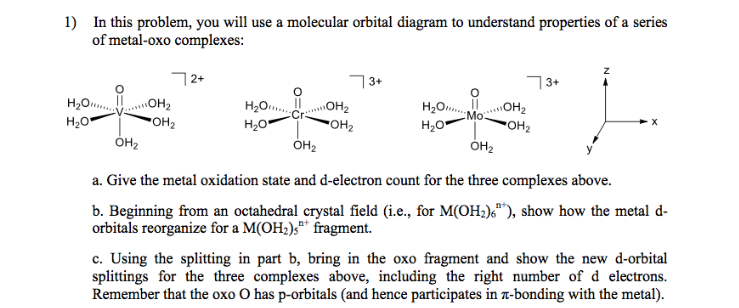
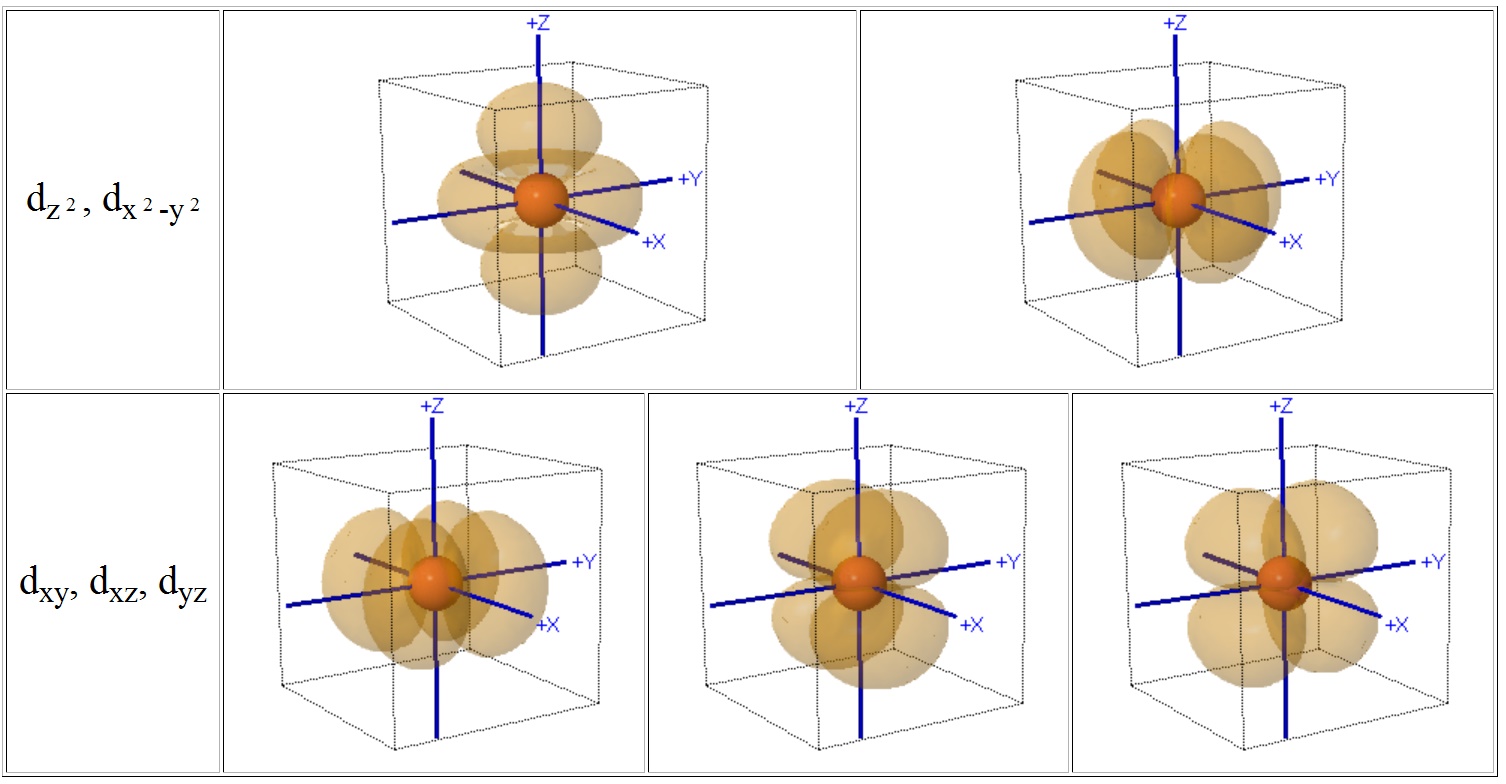
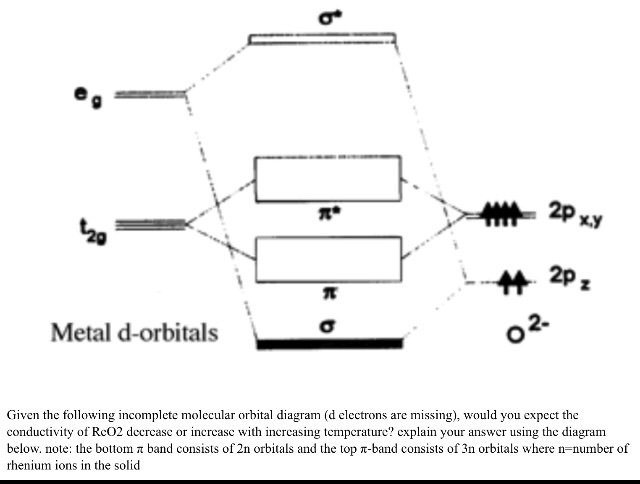
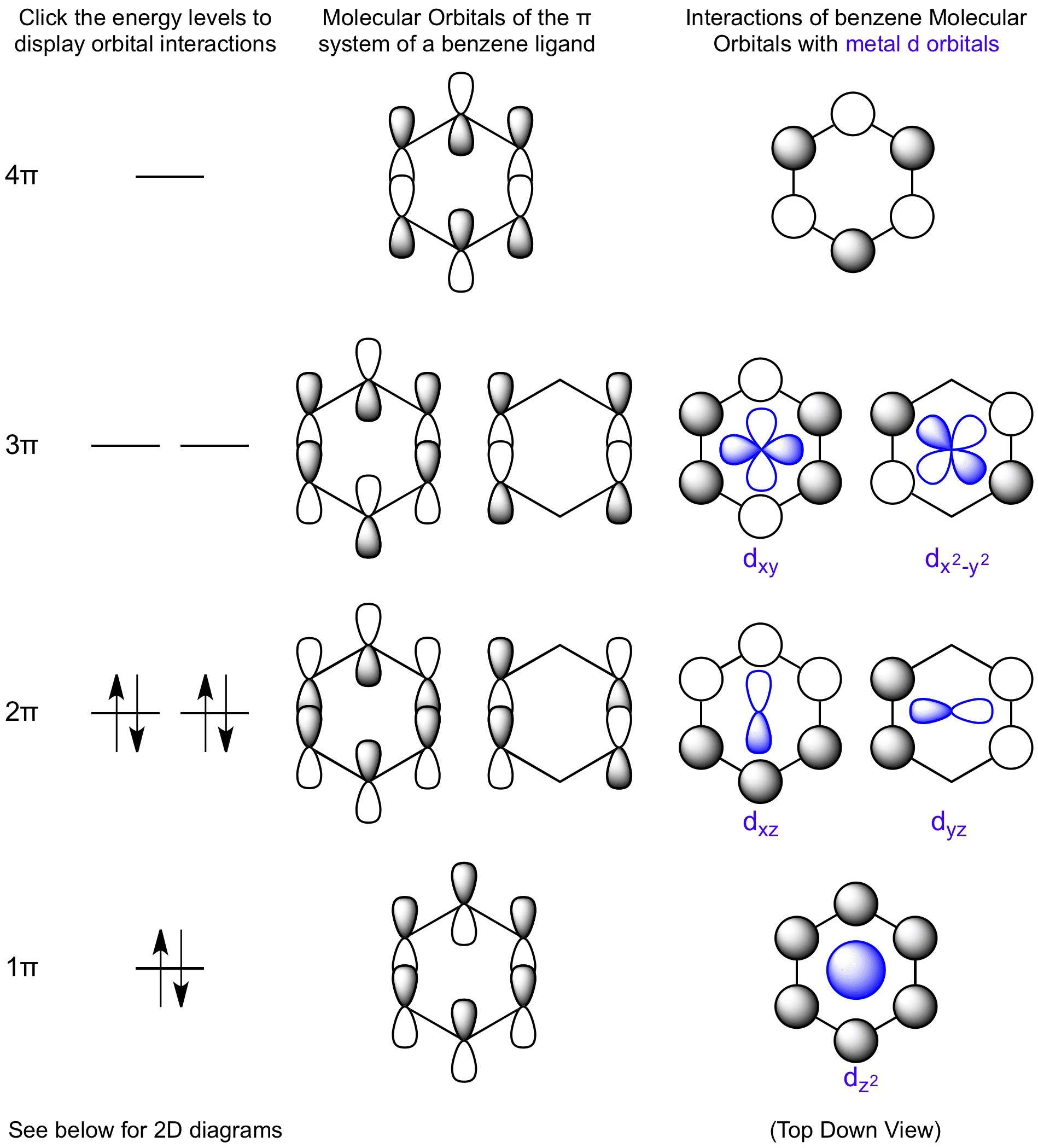
D Orbital Diagram
Here's a diagram of the first several electron configurations.
D orbital diagram. So again, it's drawn in the familiar pattern. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding figure 10. 365 molecular orbital diagram key draw molecular orbital diagrams for each of the following molecules or ions. Alright let's talk about orbital diagrams.
The 1s orbital, you can kind of just view it as a cloud around the nucleus. For example, here is the orbit diagram for asteroid 1 ceres. Large atomic orbital energy offsets correspond to relative molecular orbital energy offsets. Orbital diagrams are like the configuration notation just introduced, except with the spins of electrons indicated.
The orbital diagram in which 'aufbau principle' is violated, is represented by the option (b). Orbital diagrams are a visual way to show where the electrons are located within an atom. Draw out the possible bonding interaction of the orbitals from the peripheral atoms. These are from theoretical calculations based on the probability functions of the peculiar behaviour of.
- Delco 7si Alternator Wiring Diagram
- 2005 Trailblazer Wiring Harness Diagram
- Molecular Orbital For Ne2
The molecular orbital diagram for an o2 molecule would therefore ignore the 1s electrons on both the result is a slight change in the relative energies of the molecular orbitals, to give the diagram. You can optionally display the orbits of currently, there are no orbit diagrams for planetary satellites. In order to figure out where electrons go in an atom we have to follow 3 main rules. David's whizzy periodic table is a visual way of looking at the changing electron.
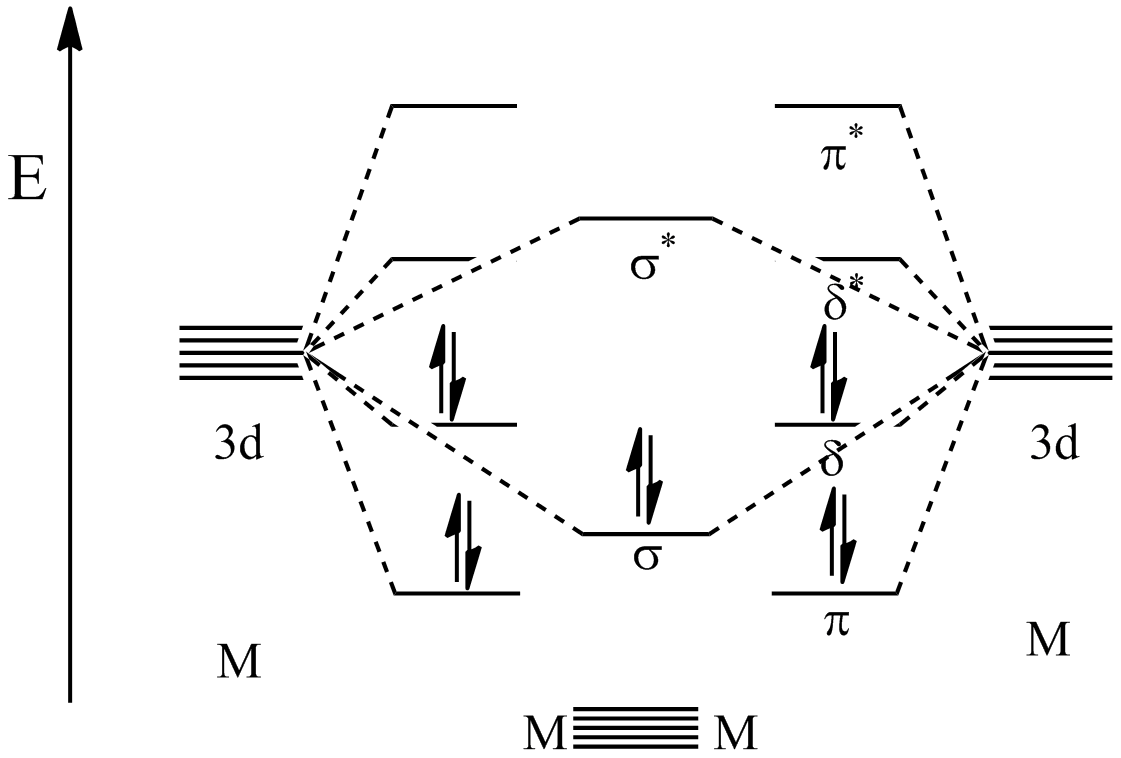
So the only possible stable configuration would be 4s2 3d3. This article explains how to create molecular orbital diagrams in latex by. The above molecular orbital diagram is for ligands which have pi antibonding orbitals too high in energy to interact with the metal orbitals. So you have your 1s orbital and it can fit two electrons, so the first electron will go into the 1s orbital and then the second electron.
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. Let's construct an mo diagram for co. According to the auf bau principle, each electron.
Orbital diagrams orbital diagrams are pictorial descriptions of the electrons in an atom. Orbital diagrams are a pictorial description of electrons in an atom. Lead (pb) has an atomic. The diagrams are not to scale and are somewhat simplified.
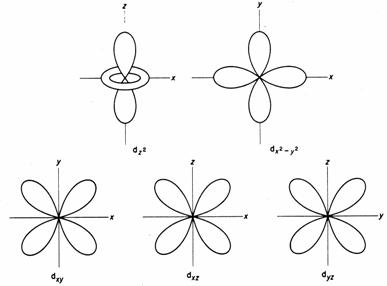
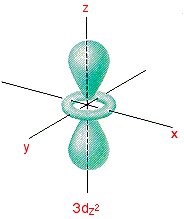
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Orbital diagrams must follow 3 rules: Start studying 3.7 orbital diagrams (chemistry). S, p and d orbital diagrams.
However, mean orbital elements of a. Determine the irreducible representation of the orbitals of the central atoms. Three rules are useful in forming orbital diagrams. In the ground state of the atoms, the orbitals are filled with electrons in order of increasing.
A box, line, or circle, is drawn to represent. Determine the bond order of each and use this to predict the stability of. The first major step is understanding the difference between two major theories: This helps with orbital energy ordering.
This is just for clarity. The effect on the molecular orbital diagram is as follows. Suppose you had a single hydrogen atom and at a particular. Orbital diagrams orbital interactions molecular orbital theory orbital energies mo diagrams hf, h2o, co2, c2h4, nh3, benzene salc hybridization symmetry and reactivity.
In this diagram (and the orbital diagrams that follow), the nucleus is shown very much larger than it really is. And in cr and cu the excites 1 electrons because by doing that both 4s and 3d orbital gains half filled i.e the stable configuration. But in science, it's pretty difficult to work with these diagrams; The molecular orbital energy diagram predicts that he2 will not be a stable molecule.
Valence bond theory and molecular. The mo diagram also shows the aos from which each. Here we have a molecular orbital diagram for the co molecule. While protons and neutrons are located in the nucleus, we've yet to touch on exactly where the electrons are in an atom.
Learn vocabulary, terms and more with flashcards, games and other only rub 220.84/month. Draw out the orbitals of the central atoms. Electron configuration + orbital diagrams. First, determine the point group of the molecule.
Use the pauli exclusion principle and hund's rule to work out how to fill shells. An mo diagram, just like an atomic orbital diagram, shows the relative energy and number of electrons in each mo. This is the orbital diagram of lead: