Valence Orbital Diagram Ground State Of Zn
Therefore as long as ground electronic state properties were the vogue, valence bond theory shone, but when the properties of these classifications written down once and for all, it is a simple matter to construct a molecular orbital energy level diagram, and with this diagram to predict the number and.
Valence orbital diagram ground state of zn. Valence electrons are those involved in forming compounds. Choose the valence orbital diagram that represents. Oxidation state and valency are one of the most fundamental properties of elements and can be studied with the help of electron configurations. Orbital diagrams and valence electrons ns.
Are the basis for the recurring pattern of 4 valence electrons. Which is most likely to be part of an ionic bond? The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Using arrows to show the spin orientation of each electron, the orbital diagram is often shown this way:
What is the electron configuration of mg? A scientifically correct term would be valence orbital to refer to the energetically accessible orbitals of an element. The partial orbital diagram below where n could be any valid quantum number. In the explanation below, i show a common means of diagramming this.
- 2006 Chrysler 300 Rear Fuse Box Diagram
- 2001 Dodge Dakota Infinity Sound System Wiring Diagram
- Ar 15 Bolt Assembly Diagram
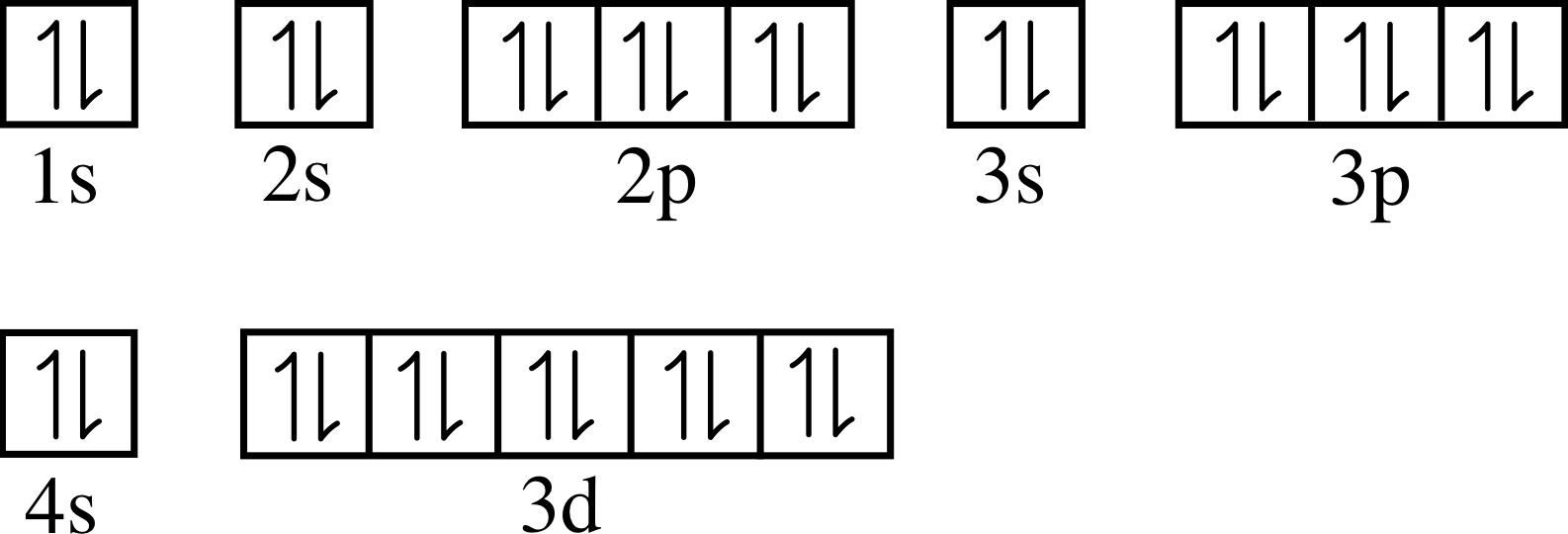
Each cluster has seven valence electrons. Draw a small box, next draw another (on the same line), next draw a set of three touching each other, draw another one and another set of three, draw another one, a set of five and put one electron pair (opposite spins) in each of the first fifteen boxes (this is the diagram for zn.) An orbital that penetrates into the region occupied by core electrons is less shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy. Transcribed image text from this question.
Orbital diagrams orbital diagrams are pictorial descriptions of the electrons in an atom. The s and p orbitals participate in the bonding of atoms with one another in covalent bonds. Carbon has a total of four valence. Figure 8.6 a vertical orbital diagram for the li ground state.
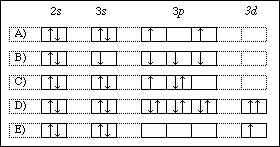
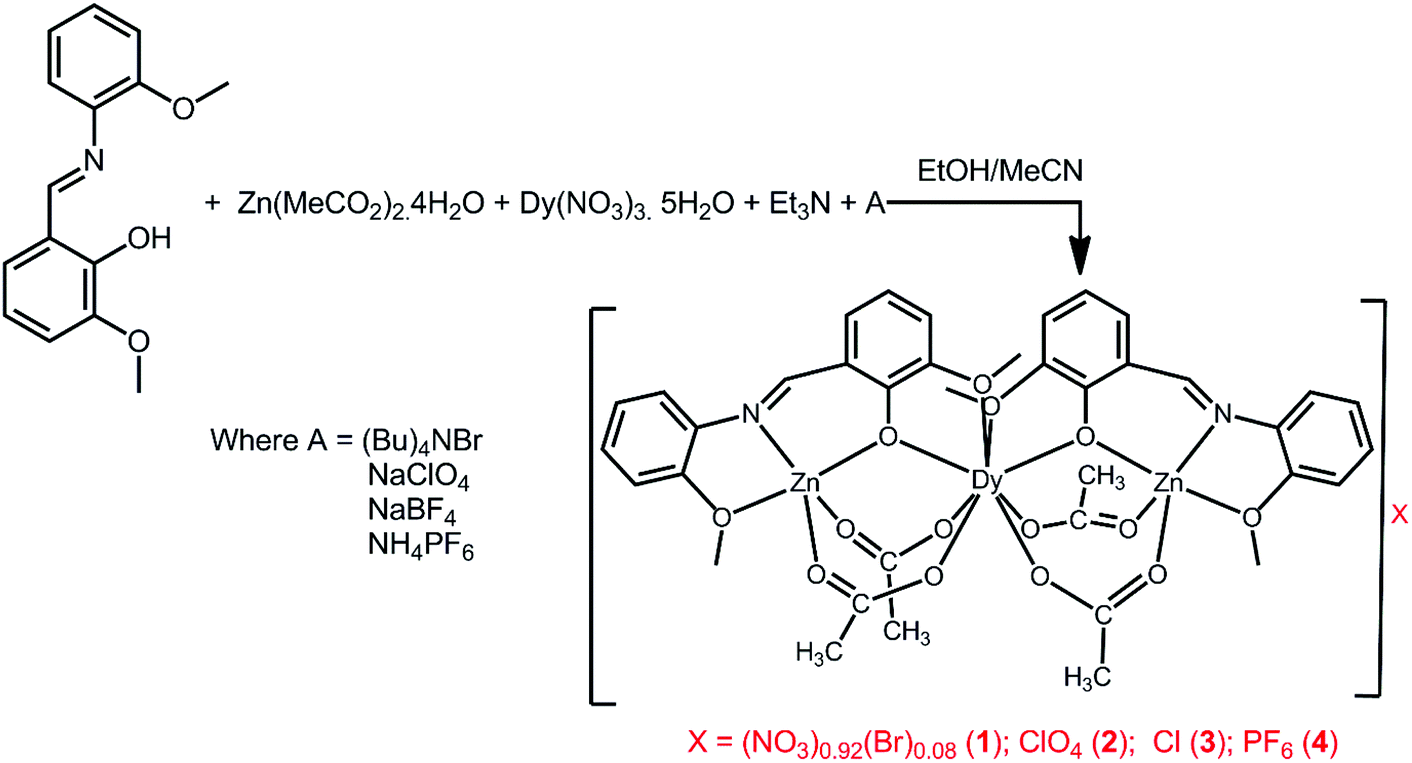
Complete the calculated mo diagram for the ground state of c2 by inserting the appropriate number of valence electrons into the appropriate orbitals. Indicate on this diagram the ground state electronic configuration of n2+ using the. Choose the valence orbital diagram that represents the ground state of zn. An atom with one the electron from a hydrogen atom drops from an excited state into the ground state.
Tags electron, valence, electron affinity, periodic table, best lewis structure. The ground state is 1s^2 2s^2 2p^2. One of the three boron electrons is unpaired in the ground state. Chemists use spdf notation and orbital box diagrams to symbolize the ground state ground state electron configurations of the elements.
Valence electrons valence electrons are the outer electrons that are involved in bonding. For main group elements, the valence electrons are the. Orbital diagrams make use of a box, circle, or line for each orbital in the energy level. Theoretical studies of valence orbital binding energies in solid zinc sulfide, zinc oxide, and zinc fluoride.
Valence bond theory states that overlap between two atomic orbitals forms a covalent bond between two atoms. States that all orbitals in the same sublevel must hold one electron before electrons will pair up in the same orbital. Orbital diagram, electron configuration, and the noble gas notation for a zinc (zn) atom. An atom is in its ground state when all the electrons in the atom occupy orbitals that result in the an orbital can be a wave function describing the state of a single electron in an atom (atomic orbital) the valence electron pattern for a carbon atom is [he]2s22p2.
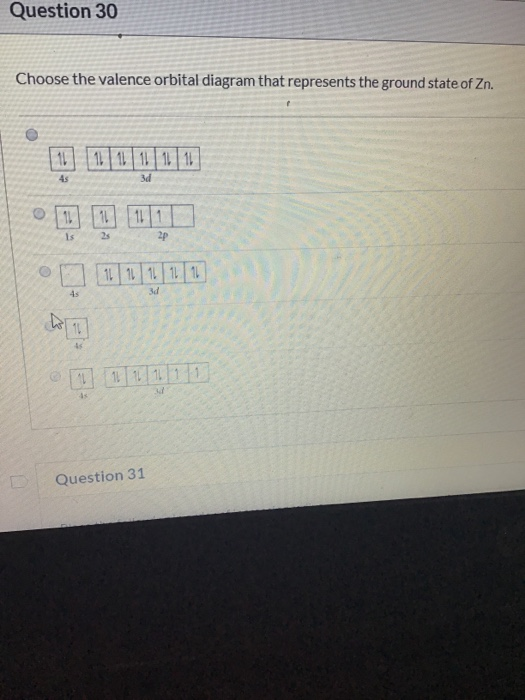
For main group elements, the orbitals with the highest n level (s and p subshells) are in the valence shell, while for transition metals. The ground state electron configuration of ground state gaseous neutral zinc is [ar].3d10.4s2 and the term symbol is 1s0. An atom with no valence electrons b. Arrow notation for electron spins.
Also what is the ground state electron configuration of zn? Electron configuration can be designated using a shorthand notation of the which set of quantum numbers correctly designates the indicated electron orbital diagram shown? These orbitals are the ones that hold the valence electrons. As you move down the periodic table, each row of elements adds another type of orbital that is available for the electrons of the atom.
The ground state electron configuration of carbon, which has a total of six electrons. 1) give the ground state electron configuration for se. The ground state electronic configuration of mg (z=12) is 1s22s22p63s2. Choose the valence orbital diagram that represents the ground state of zn.