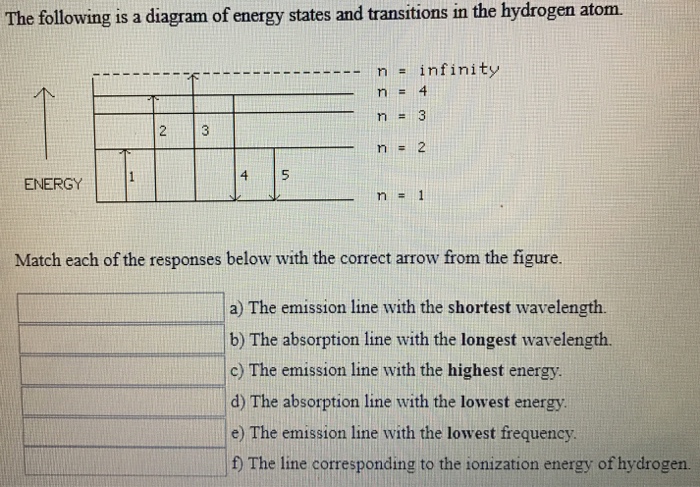
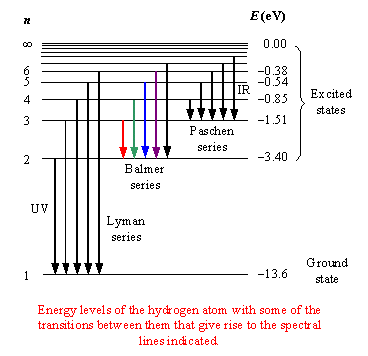
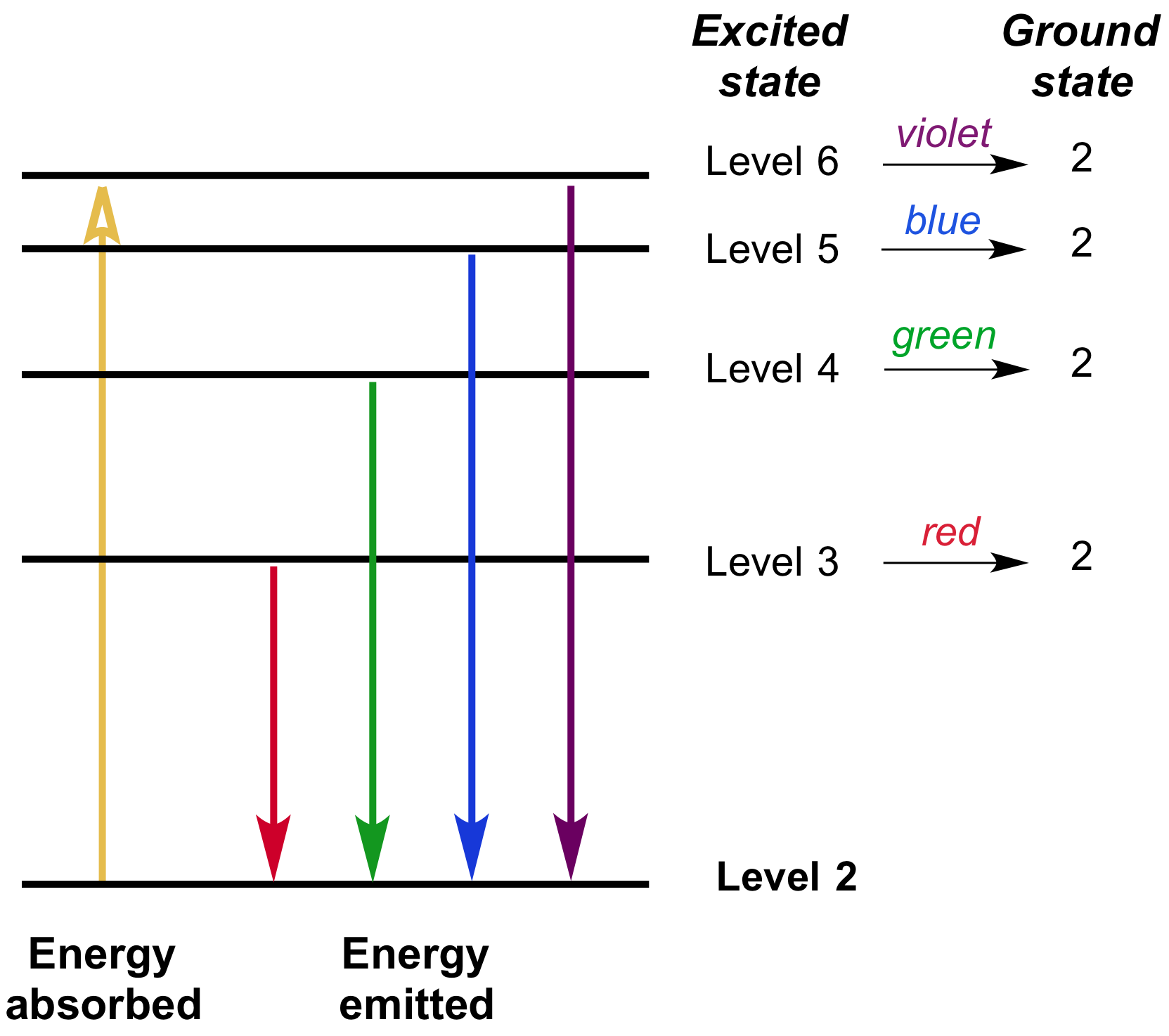
The Following Is A Diagram Of Energy States And Transitions In The Hydrogen Atom
The following is a diagram of energy states and transitions in the hydrogen atom.
The following is a diagram of energy states and transitions in the hydrogen atom. This site is using cookies under cookie policy. That means the e is independent of l and m. Energy difference is more in n.an electron of energy 11.2 ev undergoes an inelastic collision with a hydrogen atom in its ground state. To conserve energy, a photon with an.
Learn vocabulary, terms and more with flashcards, games and other study tools. For these equations which statement applies: Chemistry bohr model of the atom excited states and ground states. Start studying hydrogen energy levels.
He found that the four visible spectral lines corresponded to transitions from. The basic hydrogen energy level structure is in agreement with the bohr model. The nucleus, being much heavier than the the energy levels of the electron determine the photons that the atom will absorb or emit, allowing the powerful scientific tool of spectral analysis. Below is a diagram of the concentration of water molecules in the egg and in the solution.
The electron energy level diagram for the hydrogen atom. The hydrogen atom consists of a nucleus which is just a single proton, and an electron encircling that nucleus. According to valence bond theory, which orbitals overlap in the formation of the c−o single bond in the molecular below? Try out different models by shooting light at the atom.
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. So, you know your energy levels to be n = 5 and n = 3. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The energy of each transition is calculated using the equation e hv, where v is the frequency of each of the lines in.
Chemistry q&a library the following is a diagram of energy states and transitions in the hydrogen atom. It explains how to calculate the amount of electron transition energy that is. Thus , the energy spectrum of the hydrogen atom is discrete. Which of the following is the basis of the vsepr model of molecular bonding?
A) the emission line with the shortest wavelength. As you i just discussed in the spectral lines page, electrons fall to lower energy levels and give off light in the form of a spectrum. Amount of energy required to ionize the hydrogen atom. B) the absorption line with the.
The emission line with the longest wavelength. This chemistry video tutorial focuses on the bohr model of the hydrogen atom. The diagram for hydrogen is shown if the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. The following is a diagram of energy states and transitions in the hydrogen atom.
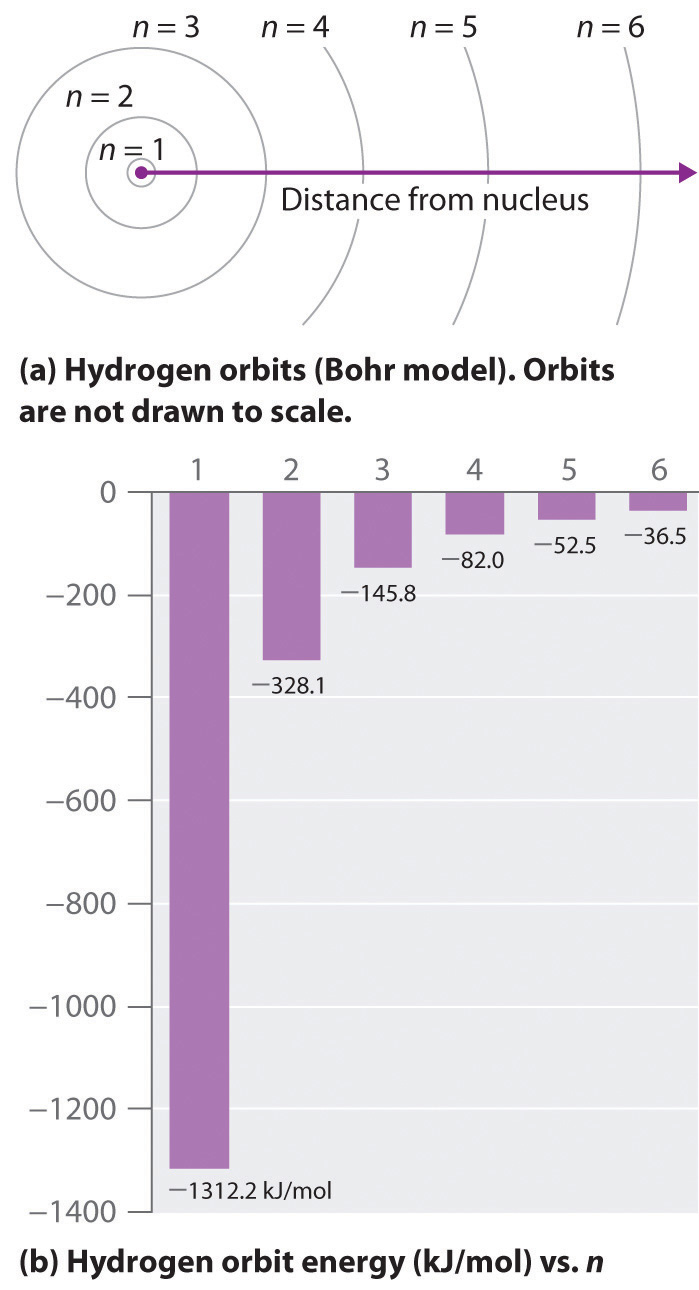
The hydrogen atom student guide. Check how the prediction of the model matches the explain the relationship between the physical picture of the orbits and the energy level diagram of an electron. Most of the individual hydrogen atoms are in the lower energy state. N (the principal quantum number), l (the angular momentum quantum well, the actual energy is just dependent on n, as you see in the following equation:
I understand, both the energy level of atom and gravitational potential energy follow the same for example suppose you are calculating the energy change in the reaction the negative sign of energy means that the energy of the electron in the atom is lower than the energy of a free electron. This is explained in the bohr model by the figure 2. The energy states of the hydrogen atom. Let me know if you have questions.
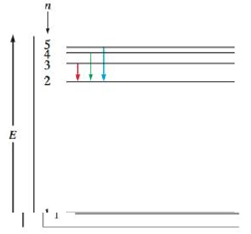
You can specify conditions of storing and accessing cookies in your browser. Infinity %3d 4 3 4 energy match each of the responses below with the correct arrow from the figure. Transcribed image text from this question. Match each arrow with the correct response below.
If light with a wavelength comparable to the when the electron is in a definite energy level we shall refer to the pn distributions as electron this completes the description of the most stable state of the hydrogen atom, the state for which n. What are the n and l quantum numbers for the electrons with the highest energy in the following atoms? When an atom loses energy, it falls from a higher energy state to a lower energy state. For е < 0 electron inside a hyperbolic the distribution of electrons in an atom the states.
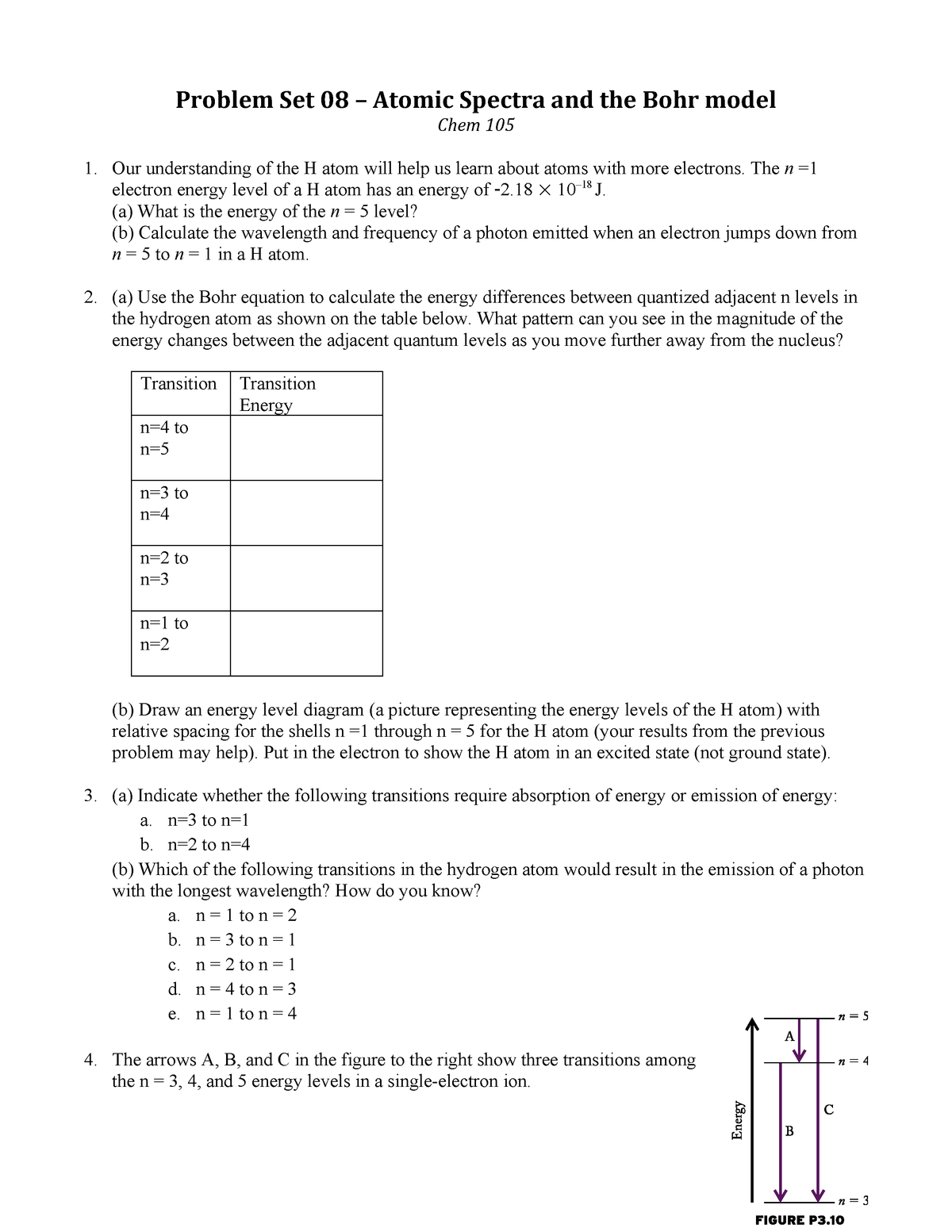
Determine whether each of the following transitions in the hydrogen atom corresponds to absorption or emission of energy. 6 to 1 (assuming 1 is ground state and 6 is 6th orbit or 5th exited state of hydrogen atom) you i was able to tell the correct answer and energy of photon in electron volts (ev) for every transition as soon as i saw these options. Rydberg's equation will allow you calculate the wavelength of the photon emitted by the the rydberg expression for an electronic transition in the hydrogen atom is Match each of the responses below with the correct arrow from the figure.
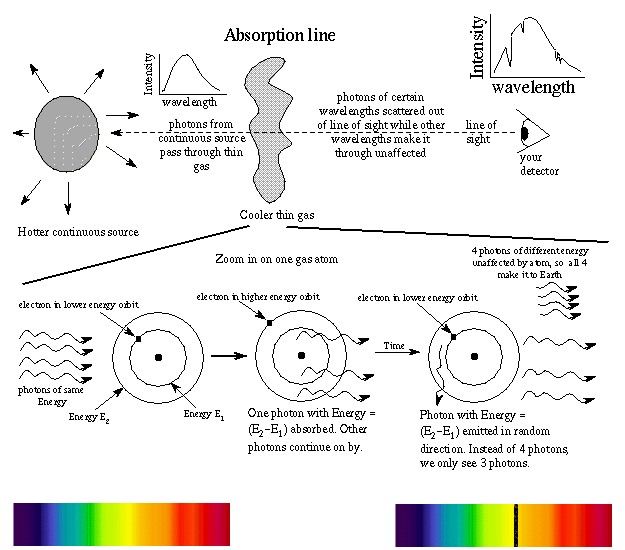
If a hydrogen atom could have any value of energy, then a continuous spectrum would have been observed when an atom in an excited state undergoes a transition to the ground state in a process called decay, it. Occasionally, a collision will supply enough energy to boost an atom into the a closed atomic electron shell is an extremely stable configuration, and by sharing their electrons, each hydrogen atom behaves as though its k. Solving for wavelength of a line in uv region of hydrogen so, if you passed a current through a tube containing hydrogen gas, the electrons in the hydrogen atoms are going to absorb energy and jump up to a. Absorption of energy or emission of energy.
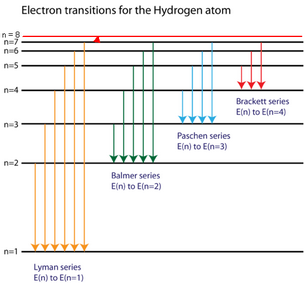
If the radial probabilities for the states are used to make sure you understand the distributions of the probability if you look at the hydrogen energy levels at extremely high resolution, you do find evidence of some. Recall that the atomic emission spectrum of hydrogen had spectral lines consisting of four different frequencies. The spontaneous (spontaneous) transitions with as in the spectra of atoms, a separate spectral line of the molecular spectrum is the result of changes. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom.
Click hereto get an answer to your question which of the following transitions in hydrogen.highest frequency emission will be of maximum energy difference. Each quantum state of the hydrogen atom is specified with three quantum numbers: Indicate which transition in a hydrogen atom would emit the photon of longer wavelength.