Orbital Diagram Of Ni
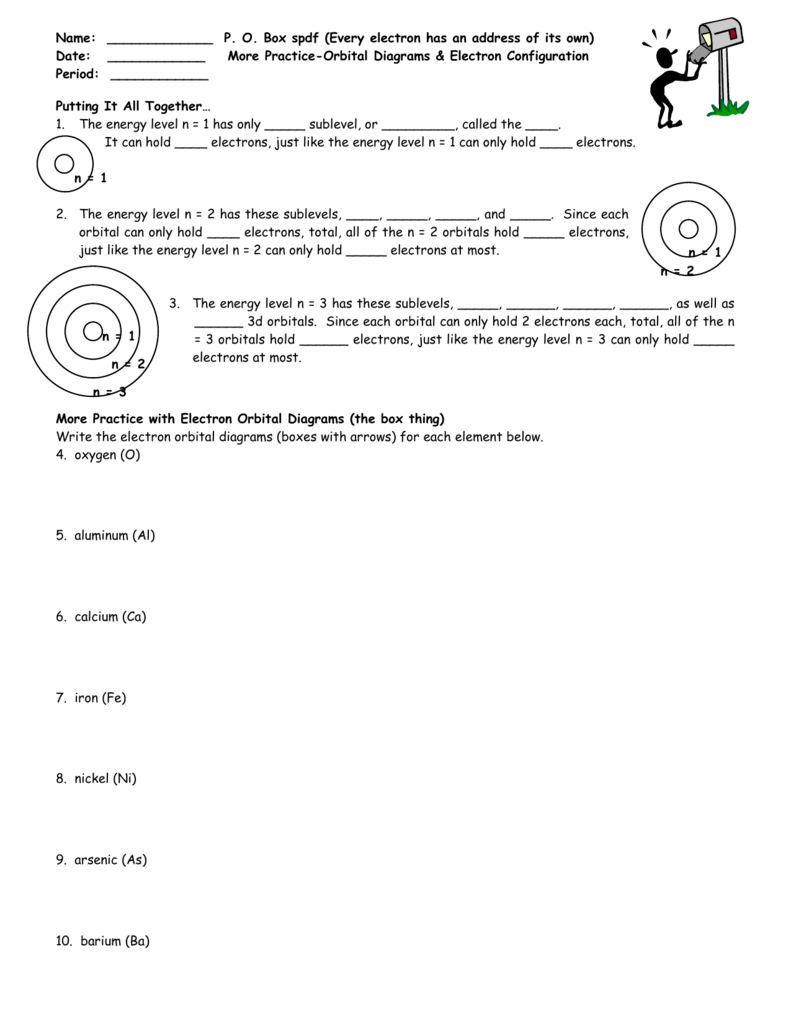
A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the aufbau principle to order the orbitals and hence.
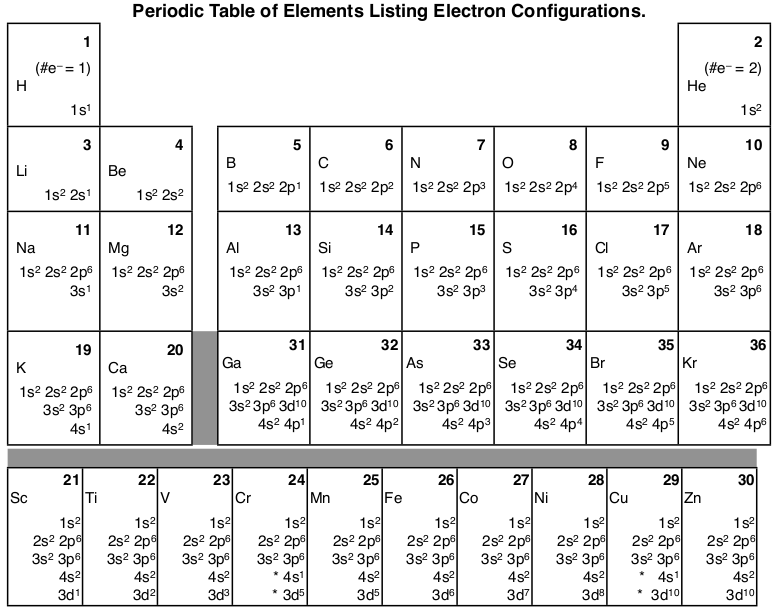
Orbital diagram of ni. 1:02:51 the organic chemistry tutor 498 268 просмотров. Orbital diagrams are a pictorial description of electrons in an atom. To show how orbital diagrams are obtained from electron configurations, consider the boron atom (z = 5). The diagram is then completed by filling the energy levels with the correct number of electrons.
Learn vocabulary, terms and more with flashcards, games and other study tools. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. These are from theoretical calculations based on each orbital, that is the space a particular quantum level occupies, can hold a maximum of two electrons of how do you work out the electron arrangement configuration for 28 nickel, ni ? Its electron configuration is ls22s22p1.
Video explanation on orbital diagrams and how to depict the electronic configuration of atoms using orbital diagrams. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. The diagrams are not to scale and are somewhat simplified. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram.
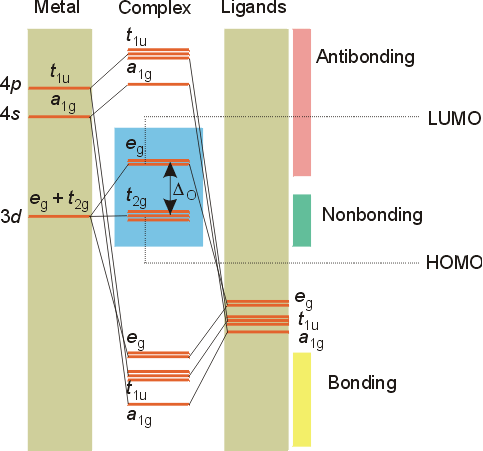
Click here to get an answer to your question molecular orbital diagram of [ni(dmg)2]. I'm going to write the electron configurations. This comic is the third of five consecutive comics published in the week before and during the solar eclipse occurring on monday, august 21, 2017 which was visible as a total solar eclipse within a band across the contiguous united states from west to east and visible as a partial eclipse across the entire. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level.
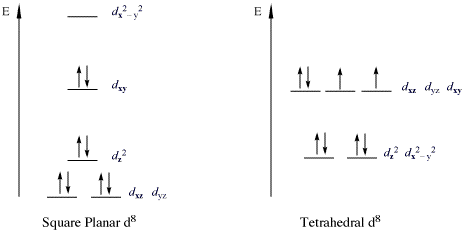
Valence bond (vb) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. The following molecules are currently available: Molecular orbital energy diagrams the relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (see the. This article explains how to create molecular orbital diagrams in latex by means of the package modiagram.
Given the rules, the orbital diagram for ni is the arrow diagram for the outermost orbitals would be: Here's a diagram of the first several electron configurations. A molecule in which all the electrons are paired, is called diamagnetic. Start studying 3.7 orbital diagrams (chemistry).
4:09 vijaya rajadurai 31 195 просмотров. | online chemistry tutorial iit, cbse chemistry, icse chemistry, engineering and medical chemistry entrance exams molecular orbital diagram of c2 molecule : In order to figure out where electrons go in an atom we have to follow 3 main rules. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom.
One showing the view looking down (or obliquely) onto the ecliptic plane. Download scientific diagram | molecular orbital diagrams for hbr and hf. The same is true of the two electrons in the 2s orbital. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Remember the number of electrons in each orbital. Below are diagrams of the planets, asteroids, and comets showing the inner solar system (out to the orbit of jupiter), the outer solar system (just beyond pluto), and the distant solar system. But this power carries a significant cost in terms of the ease with which the model can be visualized. To do 2 min read.
There are two diagrams for each region: The molecular orbitals are labeled to reflect the atomic orbitals from which they are composed as well as their symmetry properties. You can translate that to an orbital diagram, or energy diagram, as it is sometimes called. Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy to both create electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom.
This orbital energy is dependent upon the principle of the quantum number (n) as well as the azimuthal quantum number (l) which is that it depends on the shell and subshells. Construct the orbital diagram for ni. The pair of electrons in the is orbital must have opposed spins (+j, or f j). For example, to give you a glimpse at where we are headed.
More examples of orbital diagrams. Number of electrons in c2 molecule = 12. Molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms, that is, in terms of orbitals, orbital diagrams, and electron configurations. Molecules of the first row
Draw an orbital diagram of scandium?