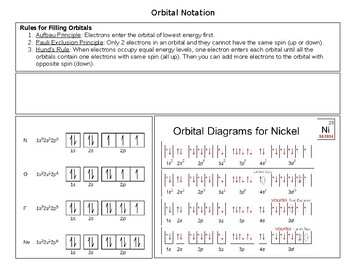
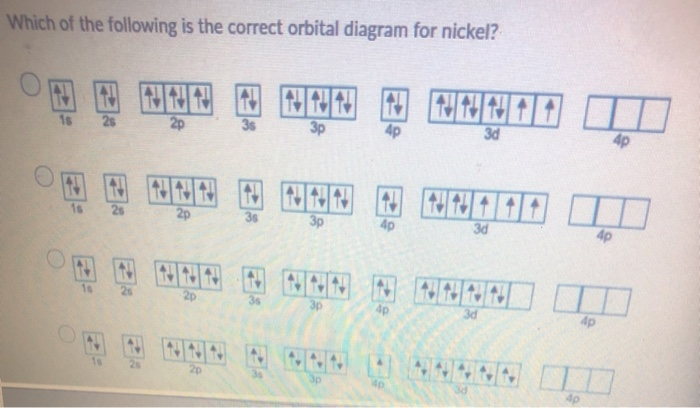
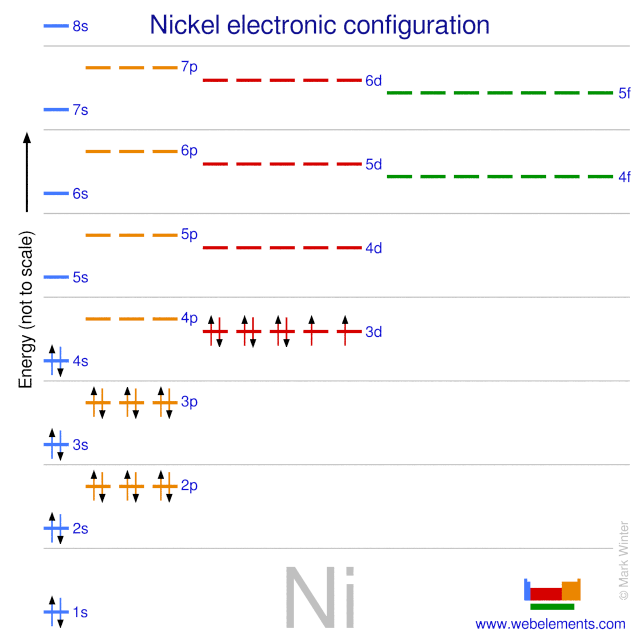
Orbital Diagram Of Nickel
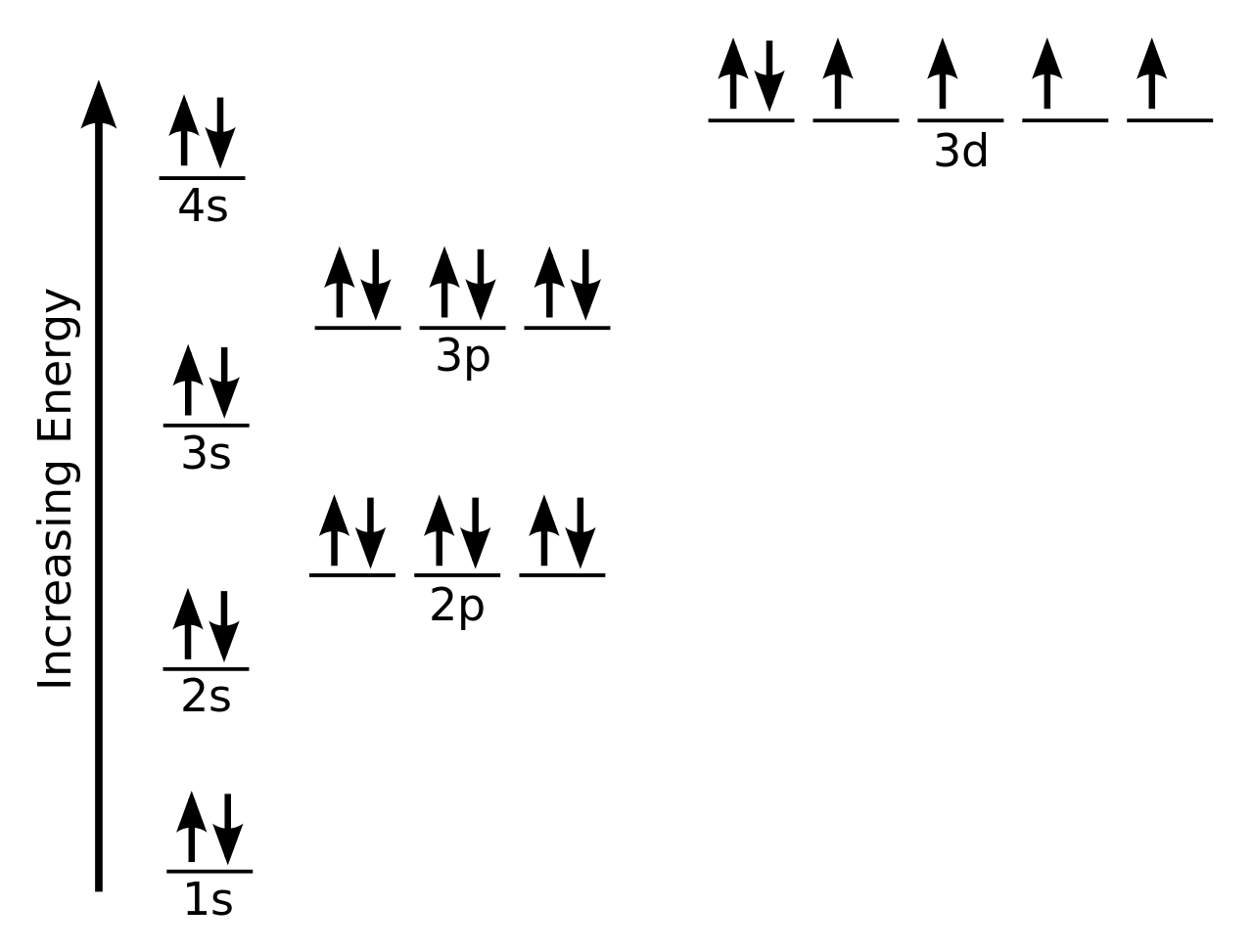
For the diagram you start with the 1 s orbital and then 2s, 2p, and so on.
Orbital diagram of nickel. Start studying 3.7 orbital diagrams (chemistry). Molecular orbital diagrams, bond order, and number of unpaired electrons draw the molecular orbital diagram for the oxygen molecule, o2. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the aufbau principle to order the orbitals and hence. Molecular orbital energy diagrams 20.
It also helps us to understand the arrangement of. Learn vocabulary, terms and more with flashcards, games and other study tools. The two spin projections are given by arrowspointing up (ms =+1/2) and down an orbital is a region of space that an electron can exist in. David's whizzy periodic table is a visual way of looking at the changing electron configuration of elements.
Orbital diagrams are a pictorial description of electrons in an atom. When molecular orbital is formed by subtraction of wave function, the type of molecular orbitals formed are called antibonding molecular orbitals and is no. The molecular orbital diagram of butadiene, and how to build it. (a) simplified orbital diagrams associated with m—l, m—x, and m—z interaction.
Analysis done by bond order. An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. Placing an electron in this orbital therefore. A mo diagram forming a mo diagram 1.
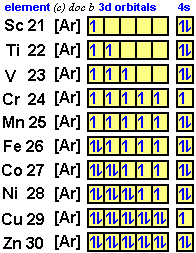
The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The filling rules are as follows: They consist of the symbol for the element in the. Therefore, it has 28 electrons in its orbitals.
9 molecular orbital diagram for co. Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy dot diagrams are very different to orbital diagrams, but they're still very easy to understand. The electronic configuration diagram represents an element in its ground state or stable state. An orbital is the quantum mechanical refinement of bohr's orbit.
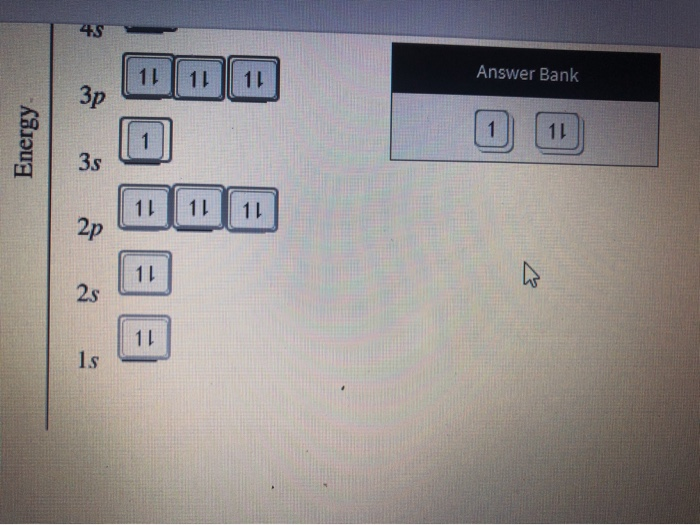
Construct the orbital diagram for nickel 1 11 1 4p 1 11 14 1 3d 1 4s answer bank 1 1 1 3p 1 1 1l 3s 1 1 1l 2p 1 2s 1s energy construct the orbital diagram of the f ion зр answer bank 3s 1 2p. Iron meteorites, or siderites, may contain iron alloyed with from 5 to nearly 20% nickel. Recall, that from the bohr model ( the maximum number of electrons that can occupy a principal energy level n is. Given the rules, the orbital diagram for ni is
It is essential that you understand the difference between them. In order to figure out where electrons go in an atom we have to follow 3 main rules. Boundary surface diagrams of the constant probability density for different orbitals help us understand the shape of orbitals. The diagrams are not to scale and are somewhat simplified.
In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space with different probabilities of having an electron. Determine the molecular shape and identify the point group of the symmetries and energy ordering are determined by examining the orbital phase pattern. Mo diagram of n2 15. Each entry has a full citation identifying its source.
The first one being the auf bau principle, the auf bau principle states that each electron occupies the lowest. Orbits and orbitals sound similar, but they have quite different meanings. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. The impossibility of drawing orbits for electrons.
Our nickel page has over 310 facts that span 105 different quantities. The boundary surface diagram for the s orbital looks like a sphere having the nucleus as its centre which in two dimensions can be seen as a circle. More examples of orbital diagrams. To plot a path for something you need to know exactly where the object is and be able to work out exactly.
If value of bond order is positive, it indicates a stable molecule and if the value is. Number of electrons in c2 molecule = 12. Wolfgang pauli states that if two electrons occupy the same orbital they must have opposite spin. (b) schematic representation of ambiphilic ligands.
Nickel is atomic number 28; A molecule in which all the electrons are paired, is called diamagnetic. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. These are from theoretical calculations based on each orbital, that is the space a particular quantum level occupies, can hold a maximum of two electrons of how do you work out the electron arrangement configuration for 28 nickel, ni ?
600 x 305 png 50 кб. | online chemistry tutorial iit, cbse chemistry, icse chemistry, engineering and medical chemistry entrance exams molecular orbital diagram of c2 molecule : From the molecular orbital diagram of n2, predict its bond order and whether it is diamagnetic or paramagnetic. 960 x 720 jpeg 79 кб.
The molecular orbital diagram of butadiene, and a system for drawing all of its pi molecular orbitals, and determining the homo and lumo. This is known as the pauli exclusion principle. Lowest energy levels fill first. Alright let's talk about orbital diagrams.
Nickel is found as a constituent in most meteorites and often serves as one of the criteria for distinguishing a meteorite from other minerals.