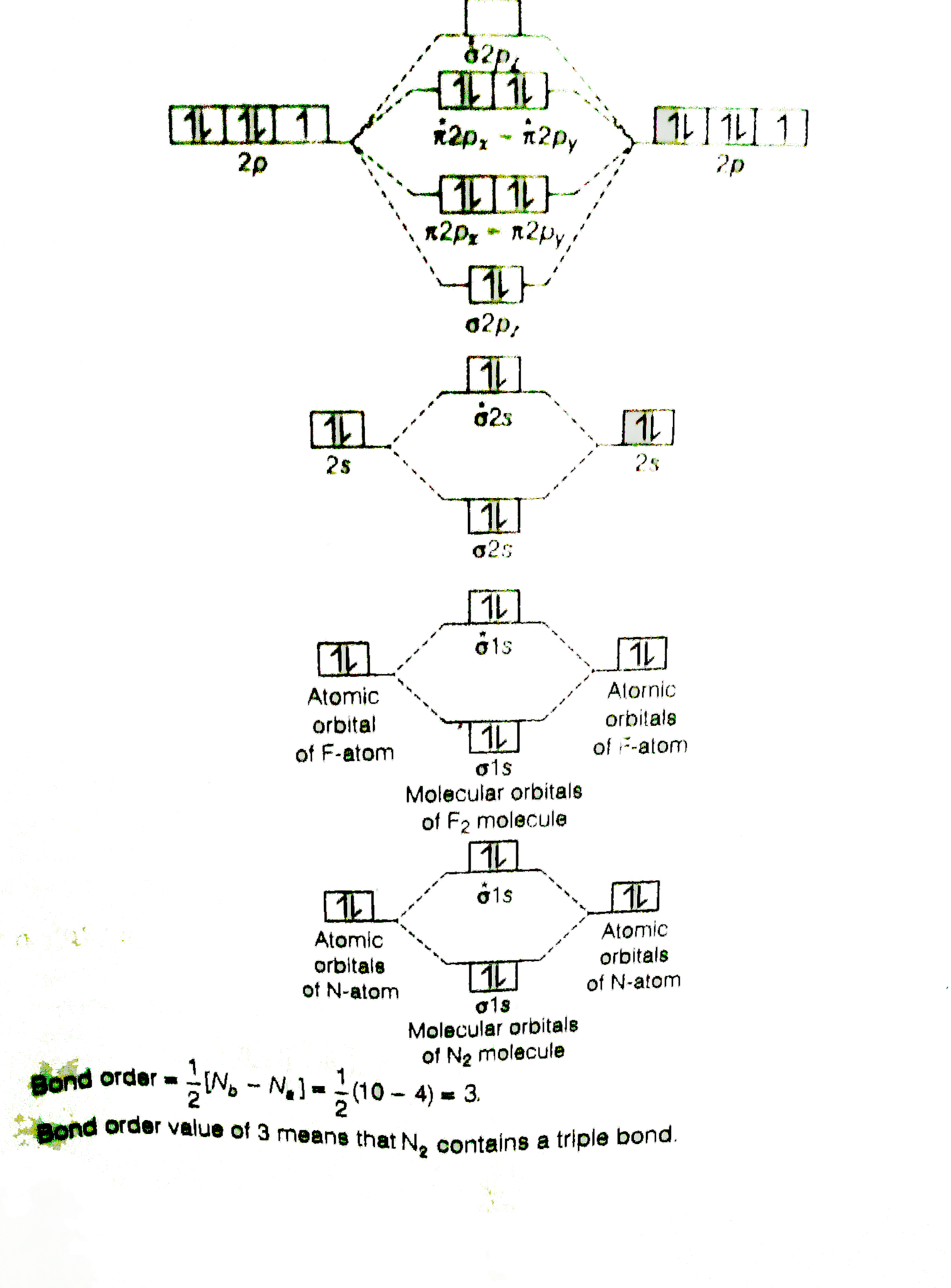Ne2 Molecular Orbital Diagram
We use the following procedure when drawing molecular orbital diagrams.
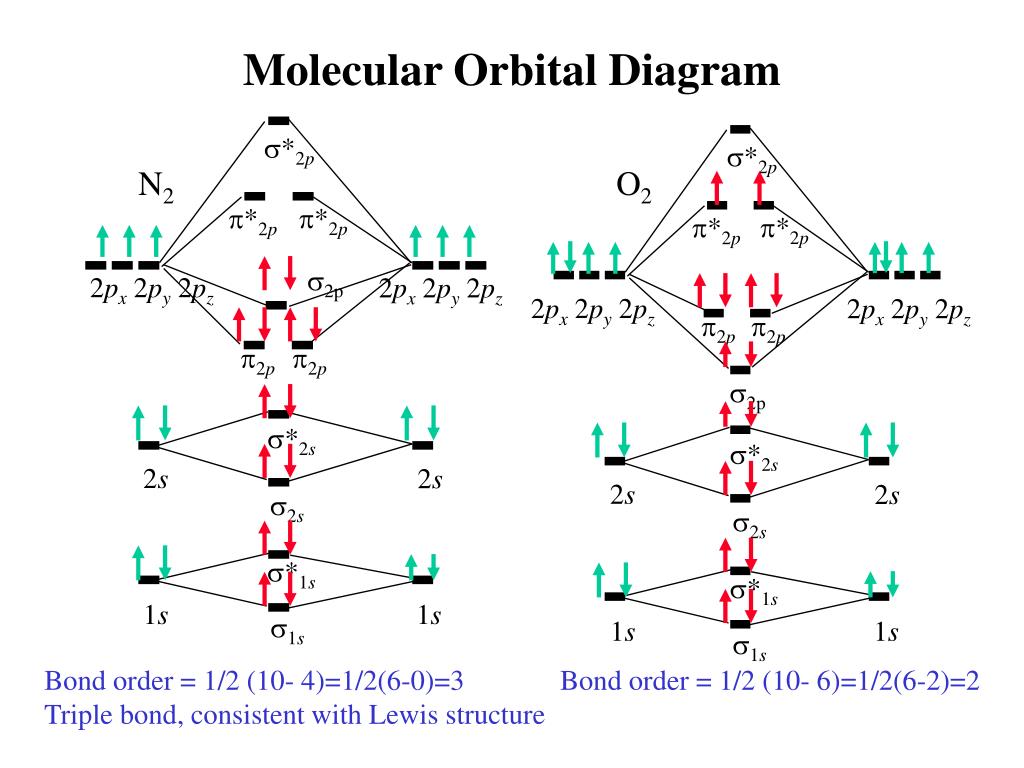
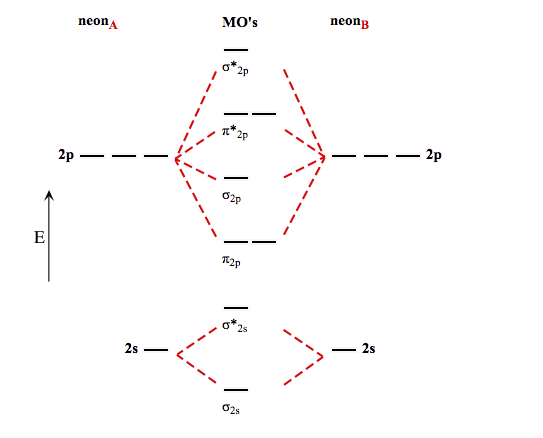
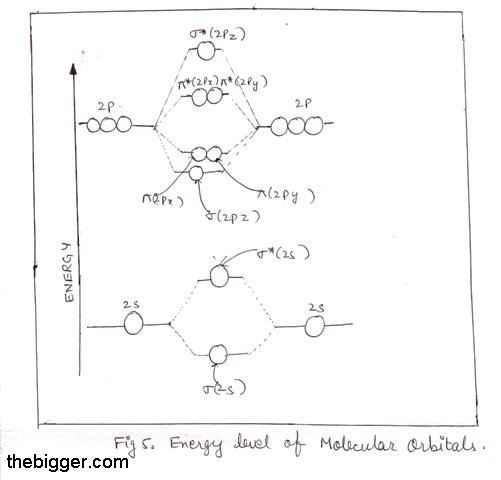
Ne2 molecular orbital diagram. Neon atom has 10 electrons and its electronic configuration is. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Each molecular orbital can only have 2 electrons, each with an opposite spin. We can see this by a consideration of the molecular electron configurations (table 3).
Why is it that the number of atomic orbitals used to generate molecular orbitals is equal to the number of molecular. According to the molecular orbital theory, the general molecular orbital configuration will be, as there are 7 electrons present in nitrogen. 168 chapter 8 & 9 covalent bonding and molecular structures we can describe diatomic molecules the molecular orbital diagram predicts co to be very stable with a bond order of three. The video below describes how to generate molecular orbital diagrams for b₂ and other diatomic molecules from row 2 elements of the.
However, we can predict that the be2 molecule and the ne2 molecule would not be stable. Considers bonds as localized between one pair of atoms. Will the mo diagram be the same as that of $\ce{n2}$ or not? Let me explain the molecular orbital diagram of n2 using its diagram.
- Poulan Pro Drive Belt Diagram
- 2008 Super Duty Mirror Wiring Diagram
- Century Ac Motor Wiring Diagram 115 Volts
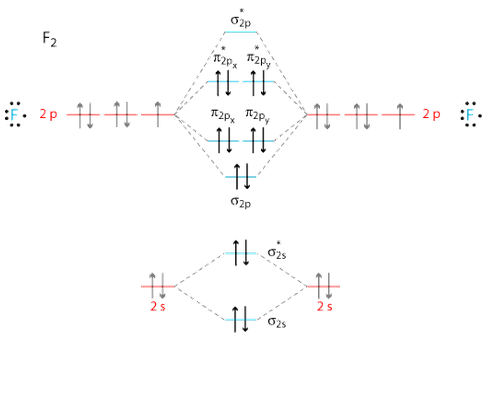
The molecular orbital (mo) theory is a way of looking at the structure of a molecule by using molecular orbitals that belong to the molecule as a whole. The molecular orbital diagram shows the creation and filling of mos in a bond. The molecular orbital diagram of are shown below. As it can be seen from the mot of o2 , the electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature.
Molecular orbital diagram of c2 molecule : Valence bond theory and molecular orbital theory. Individual atomic orbitals ao are arranged on the far left and far right of the diagram. Valence bond theory proposes that electrons are.
There are two types of molecular orbitals that can form from the overlap of two atomic s orbitals figure 8.34 this is the molecular orbital diagram for the homonuclear diatomic. If ne 2 did form, it would be diamagnetic. Mo diagram of homonuclear diatomic molecules. This article explains how to create molecular orbital diagrams in latex by means of the package modiagram.
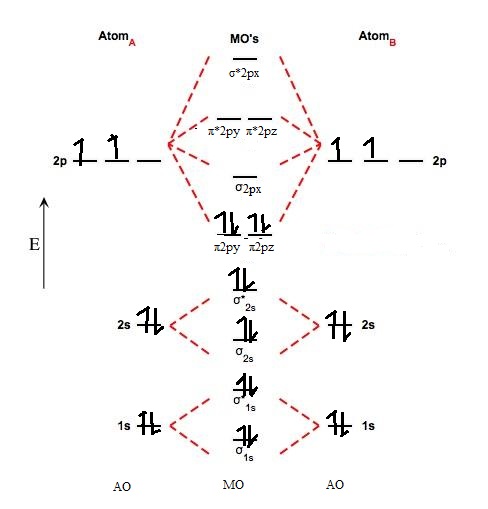
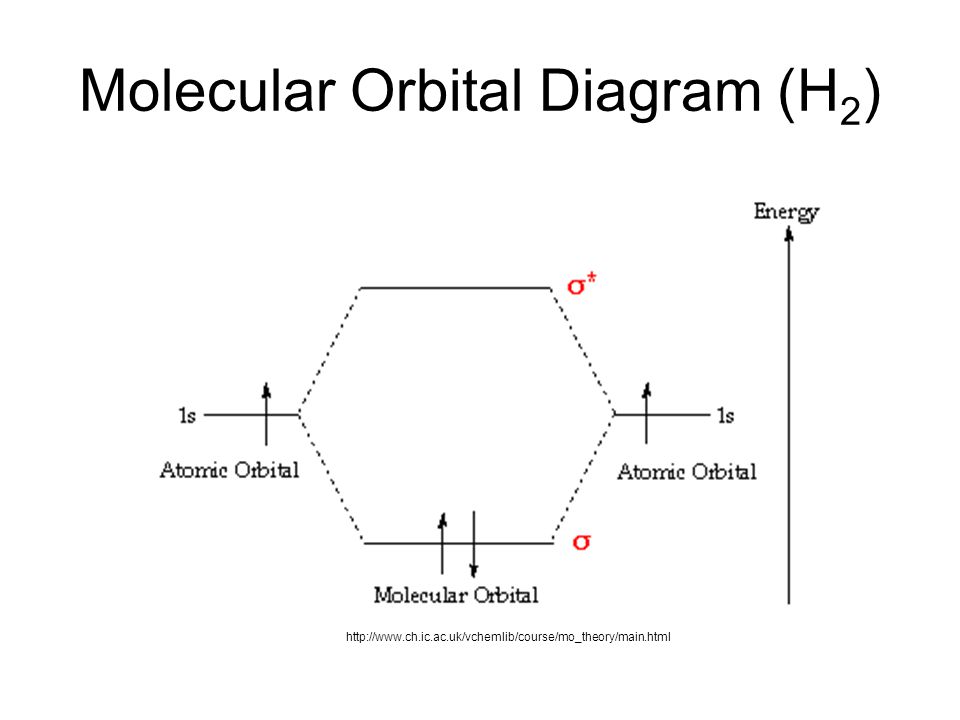
Transformational properties of atomic orbitals. 1s 1s 2s 2p 2p 2s 2p 2p 2p 2p we predict the nitrogen. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h2+. (b) the shapes of the molecular orbitals are obtained by squaring the.
The first major step is understanding the difference between two major theories: Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2). What will be the molecular orbital diagram for nitrite ion? The following molecules are currently available:
Now note that even in this advanced molecular orbital theory a bunch of approximations is introduced, and the answer in general depends on at which level of theory calculations are done. Eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Within the diagram, orbitals are represented by horizontal lines. Among those elements there are some traits that are always true
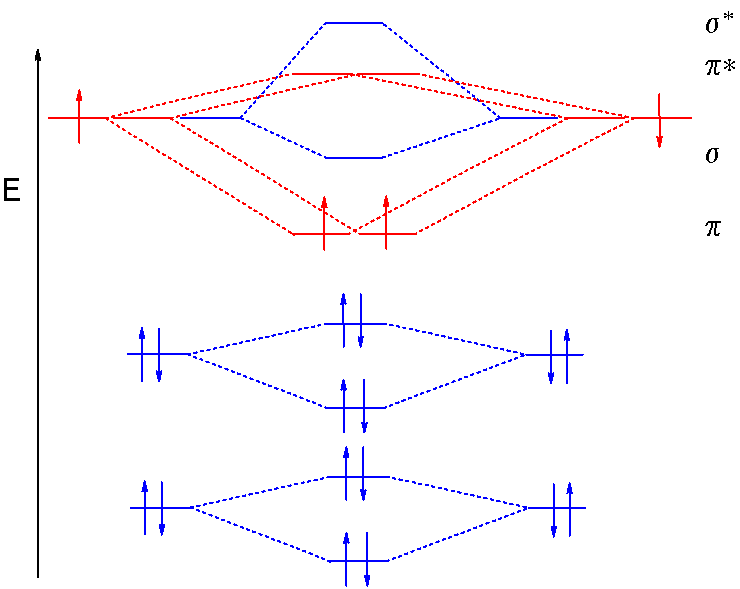
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the. There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). The diagram is then completed by filling the energy levels with the correct number of electrons. The other is for after nitrogen.
• when bonds are formed, atomic orbitals combine according to their symmetry. This second orbital is therefore called an antibonding orbital. We assume that the electrons would fill the molecular orbitals of molecules like electrons fill atomic we will use this diagram to describe o2, f2, ne2, co, and no. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram.
Molecules of the first row Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. As the bond order value for molecule is zero, it is unstable and cannot exist.
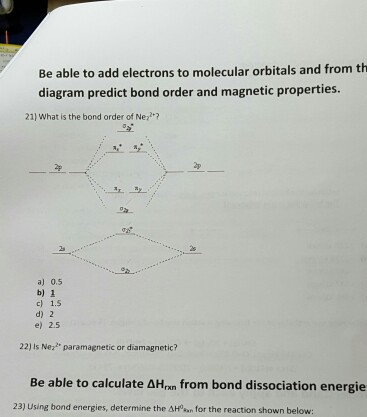
A molecule in which all the electrons are paired, is called diamagnetic. Ne2 molecular orbital diagram posted on may 15, 2016 by admin a diagram is shown that has an upward facing vertical arrow running along the left 9 7 ordering molecular orbitals according to energies fill the total electrons in respective orbitals for o2 f2 and ne2 then calculate their bond order no of. How does the directional nature of orbitals affect the bond strength? Is h2 a viable molecule for the molecular orbital theory?
Total # of bonding electrons. The bond order of is, 3. Molecular orbital diagram for nitrogen gas ( 1 ion) (n2( )). B, c, n, o, f, ne.
Number of electrons in c2 molecule = 12. • because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. One is for the elements up to nitrogen.
| online chemistry tutorial iit, cbse chemistry, icse chemistry source : The orbital correlation diagram in predicts the sa. The molecular orbital volume encompasses the whole molecule. The molecular orbital diagram of hypothetical molecule is given in the attachment.
Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the pauli principle, the what is the molecular orbital diagram for the diatomic neon molecule, ne2? Fill from the bottom up, with 9 valence electrons total.