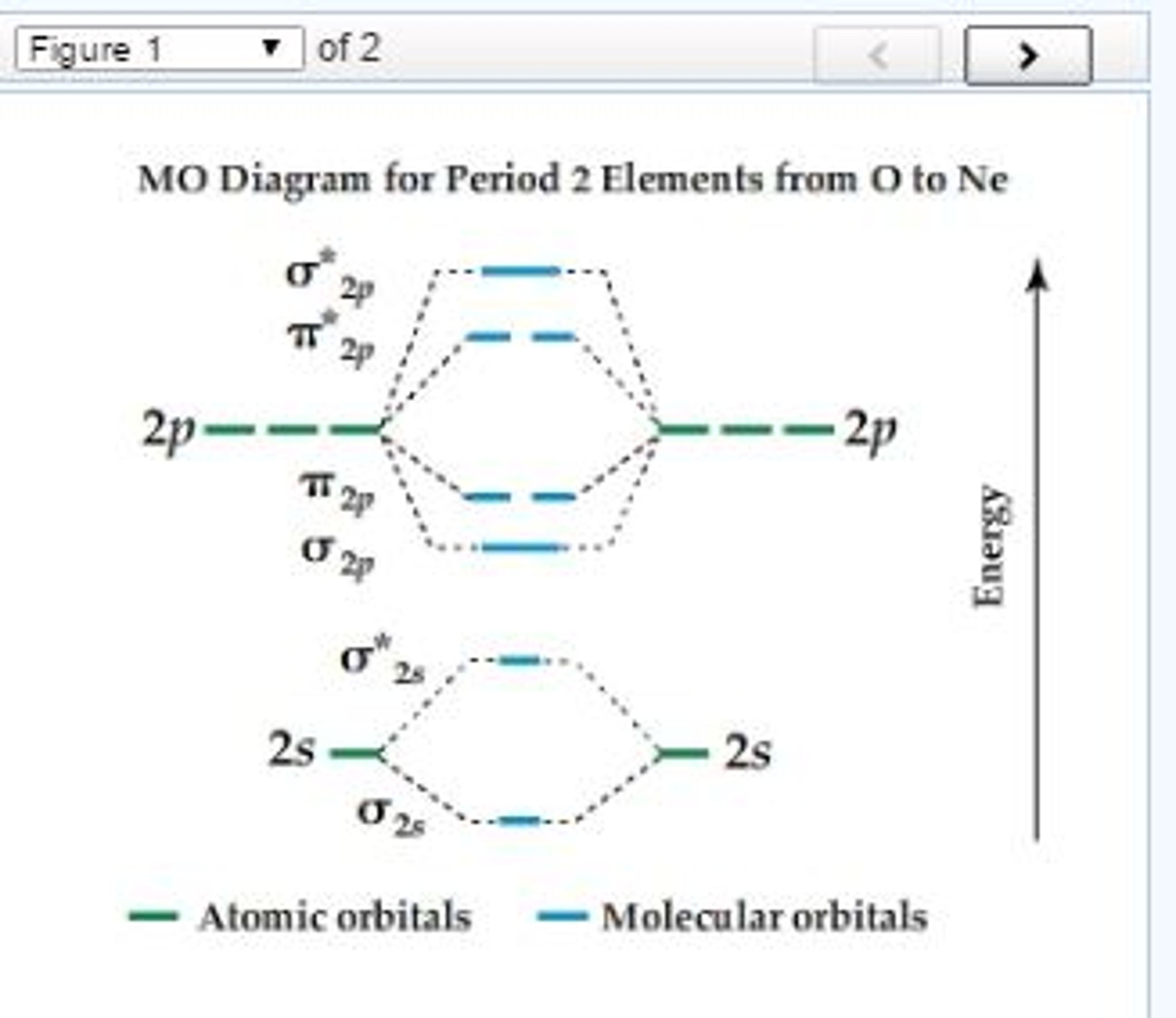
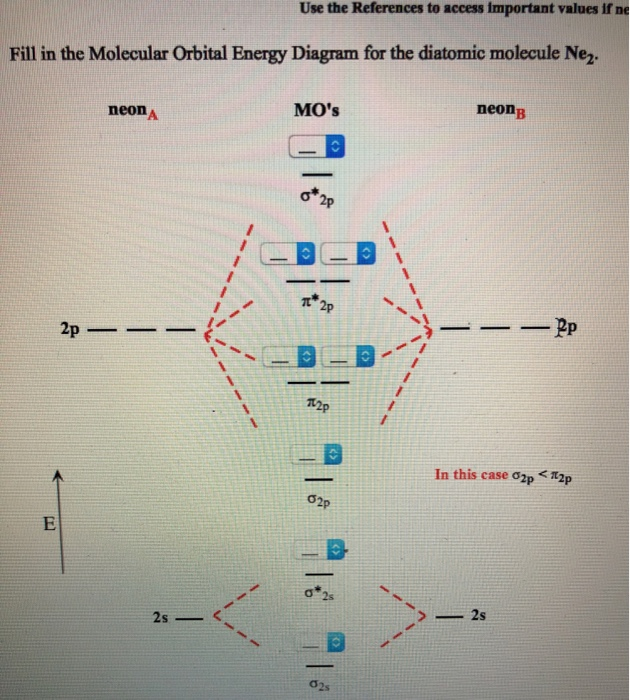
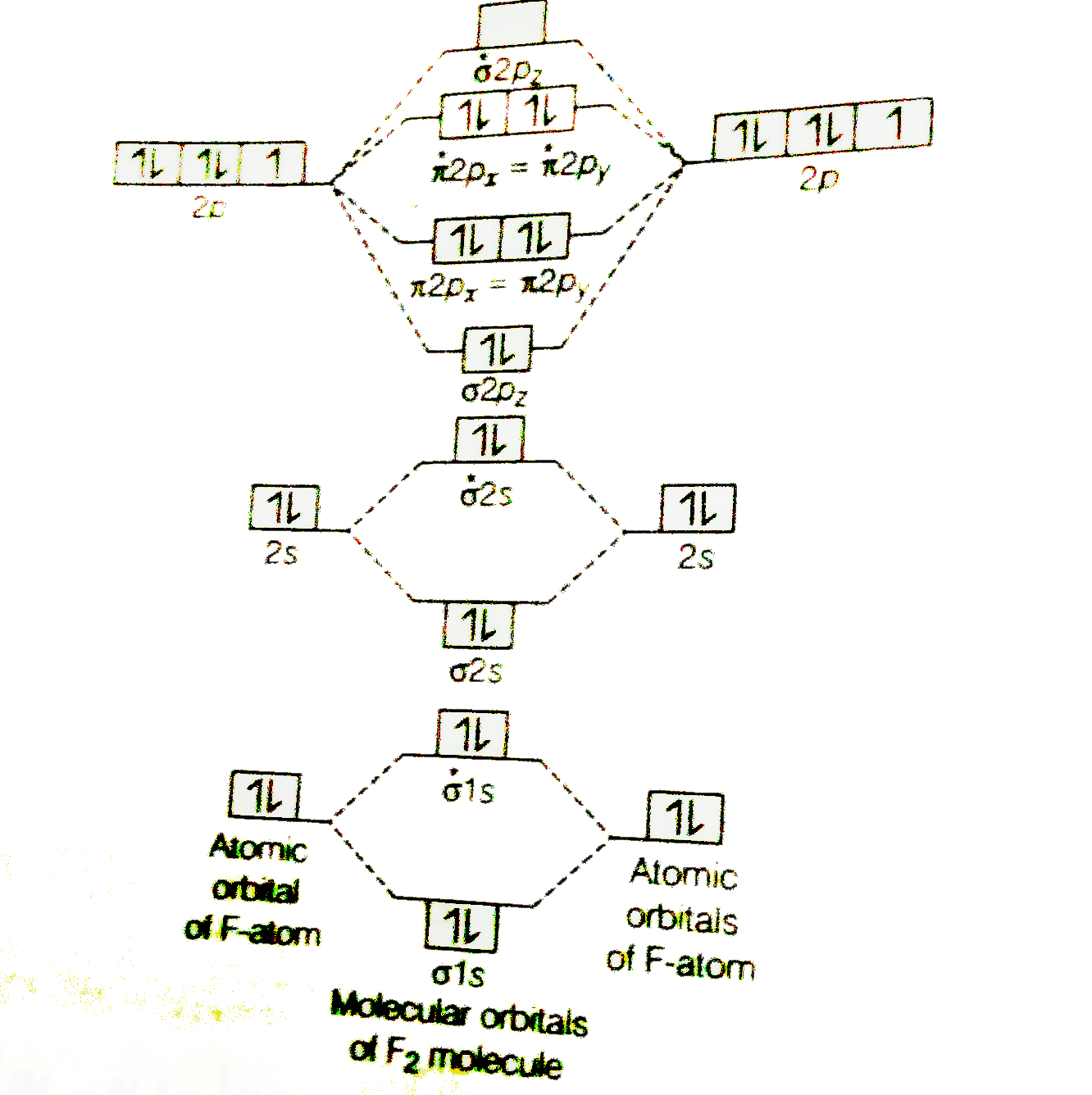
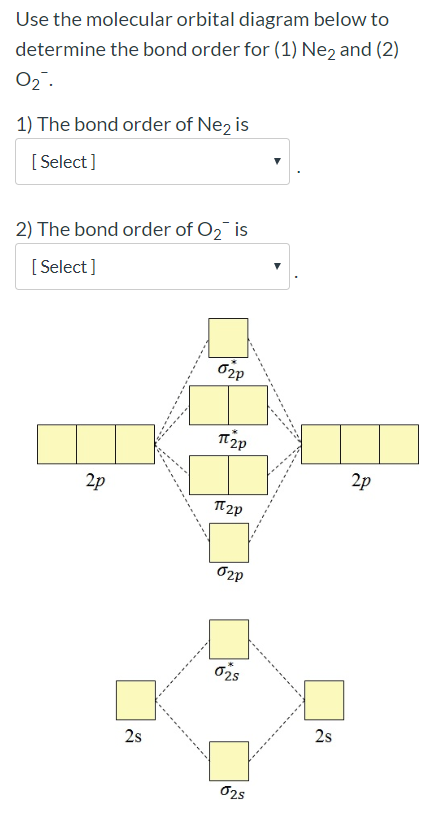
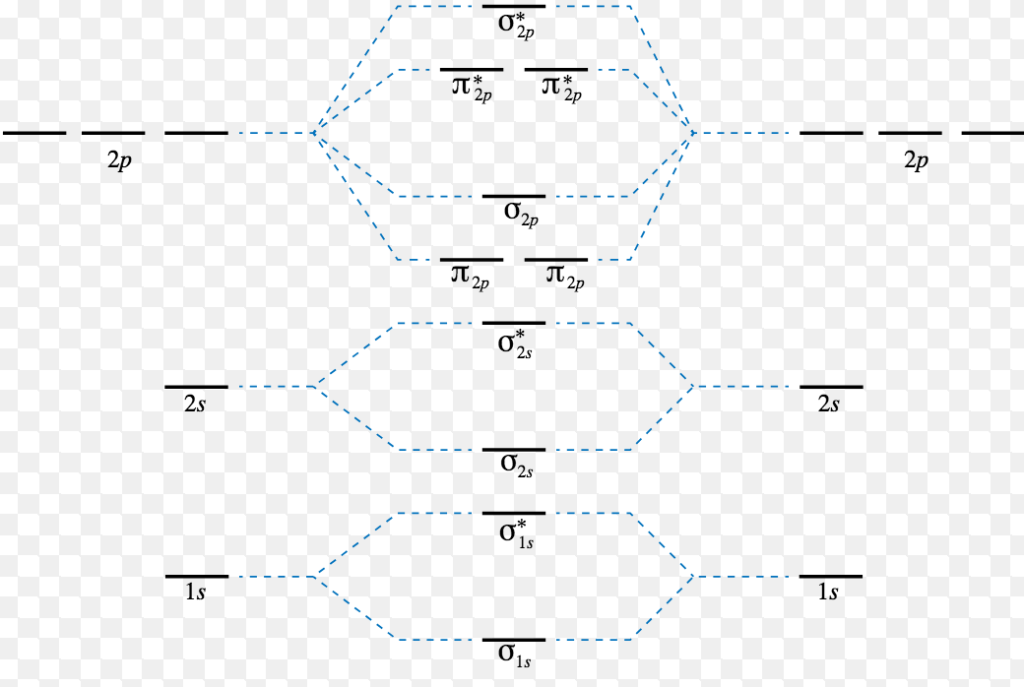
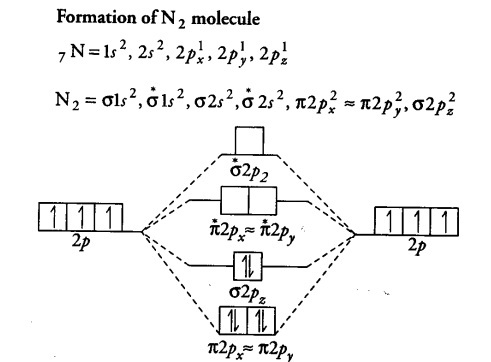
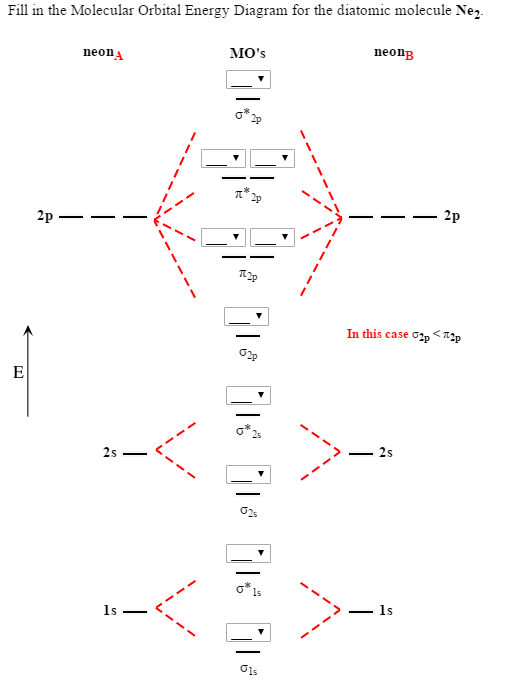
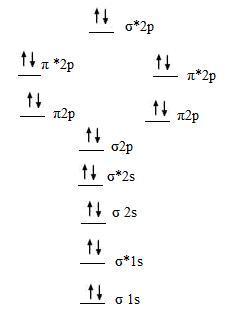Molecular Orbital Diagram For Ne2
Be sure your diagram contains all of the electrons in the ion, including any core electrons.
Molecular orbital diagram for ne2. We use the following procedure when drawing molecular orbital diagrams. Part a by drawing molecular orbital diagrams for b2, c2, n2, o2, and f2, predict item 2: Chemistry · 1 decade ago. One is for the elements up to nitrogen.
The other is for after nitrogen. An mo diagram, just like an atomic orbital diagram, shows the relative energy and number of electrons in each mo. We can see this by a consideration of the molecular electron configurations (table 8.3). Neon atom has 10 electrons and its electronic configuration is.
Then we rank them in order of increasing energy. The orbital correlation diagram in predicts the sa. Seeing a molecular orbital diagram for n2 will clarify what i mean. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
- 2004 Dodge 2500 Trailer Wiring Diagram
- Craftsman Lt2000 Drive Belt Installation
- Honda Gx390 Charging System Wiring Diagram
A molecule in which all the electrons are paired, is called diamagnetic. One is for the elements up to nitrogen. Part e apply molecular orbital theory to determine the bond order of ne2. There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc).
What is the orbital diagram for nitric oxide?? Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the pauli principle, the what is the molecular orbital diagram for the diatomic neon molecule, ne2? Each molecular orbital can only have 2 electrons, each with an opposite spin. Mo occupancy and molecular properties for b2 through ne2.
Now note that even in this advanced molecular orbital theory a bunch of approximations is introduced. Heteronuclear diatomic molecules how do heteronuclear mos. There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). However, we can predict that the be2 molecule and the ne2 molecule would not be stable.
This molecular orbital model can be used to explain why he2 molecules don't exist. The molecular orbital volume encompasses the whole molecule. Learn vocabulary, terms and more with flashcards, games and other study tools. C2 12 2 0 n2 14 3 0 o2 16 2 2 f2 18 1 0 ne2 20 0 0.
Within the diagram, orbitals are represented by horizontal lines. Use molecular orbital theory to predict molecular geometry for simple triatomic systems • rationalize molecular structure for several specific systems in terms based on energy, which can interact? The molecular orbital diagram of hypothetical molecule is given in the attachment. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2).
Among those elements there are some traits that are always true Draw the molecular orbital energy level diagram for the following substances, and complete the tables. This shows the mo diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the effective nuclear charge.
Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2). We can ignore the #1s# orbitals, because they do not contain the valence electrons. The walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. Start studying molecular orbital theory.
Bellywelly asked in science & mathematics. B, c, n, o, f, ne. Combining a pair of helium atoms with 1s2 electron configurations would the molecular orbital diagram for an o2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the. Ne2 molecular orbital diagram posted on may 15, 2016 by admin a diagram is shown that has an upward facing vertical arrow running along the left 9 7 ordering molecular orbitals according to energies fill the total electrons in respective orbitals for o2 f2 and ne2 then calculate their bond order no of.
All this is simply because the primitive molecular orbital theory does not explain the things, rather it rationalizes them (for differences between explanation and rationalization, please, read here). What is the molecular orbital diagram for no2? Of molecular orbitals changes for diatomicmolecules like o2, f2, ne2 is 1s 1s 2s 2s 2pz2px = 2py)( *2px= *2py) 2pzbond order (b.o.) is defined as one half the difference between the numberof electrons present in the bonding and the. 2p 2s mo diagram for n2 n2, o2, f2, ne2?
We assume that the electrons would fill the molecular orbitals of molecules like electrons fill atomic we will use this diagram to describe o2, f2, ne2, co, and no. The first major step is understanding the difference between two major theories if you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it. | online chemistry tutorial iit, cbse chemistry, icse chemistry, engineering and medical chemistry entrance exams molecular orbital diagram of c2 molecule : The molecular orbital diagram shows the creation and filling of mos in a bond.
Transformational properties of atomic orbitals. Looking at ne2 molecular orbitals, we see that the order is consistent with the generic diagram shown in the previous section. Write the ground state molecular orbital electron contiguration | molecule enetsy level diagram including each atom's energy levels) molecular orbital clectron. Molecular orbital diagram for no?
Number of electrons in c2 molecule = 12. Individual atomic orbitals ao are arranged on the far left and far right of the diagram. Looking at ne2 molecular orbitals, we see that the order is consistent with the generic diagram shown in the previous section. Answered october 13, 2019 · author has 6.2k answers and 2.8m answer views.
As the bond order value for molecule is zero, it is unstable and cannot exist. How to write simple molecular orbital diagrams and determine the bond order. The orbital energies decrease across the period as the effective. The molecular orbital (mo) theory is a way of looking at the structure of a molecule by using molecular orbitals that belong to the molecule as a whole.
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of.