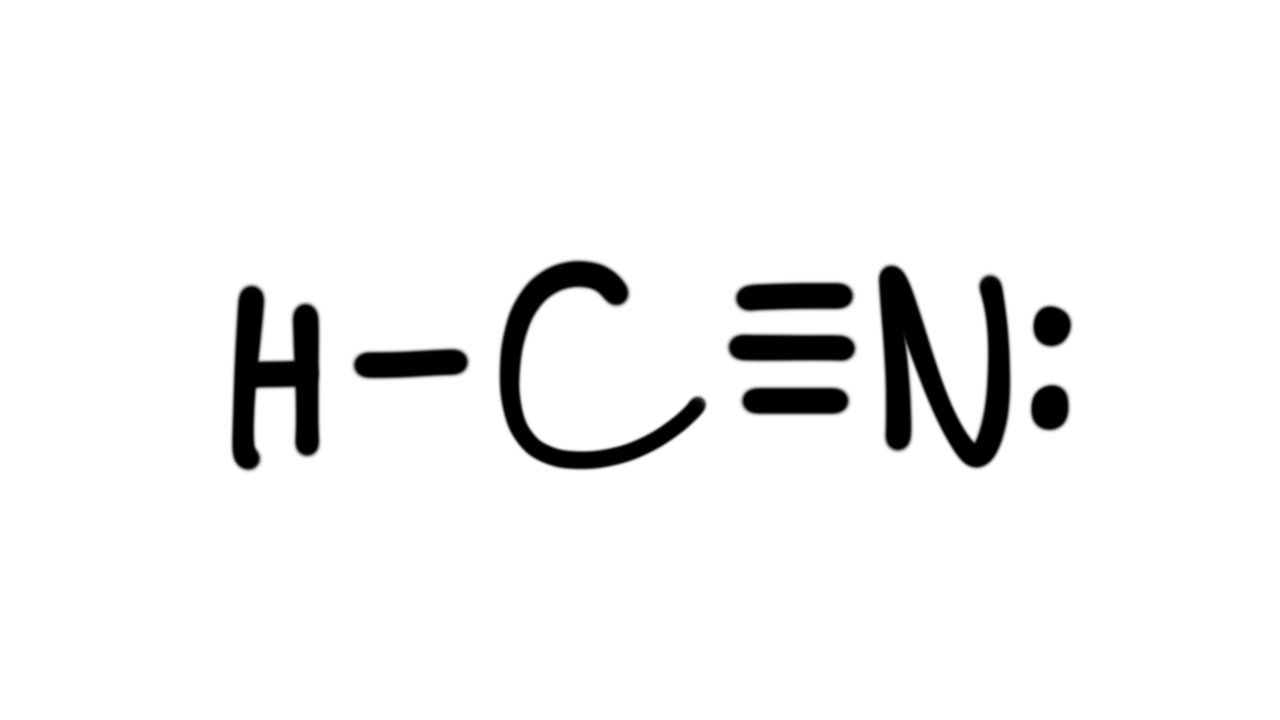Lewis Structure For Hcn
Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Lewis structure for hcn. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Let's start with the first one, water. _ lab partner_ lab section_ lab report for vsepr theory and shapes of molecules hcn 2. The electronic structure of molecules can be illustrated by lewis structures, which can be used to and properties such as geometry, bond orders, bond lengths the following are the geometries that will be adopted by atoms with n atoms/lone pairs around them:
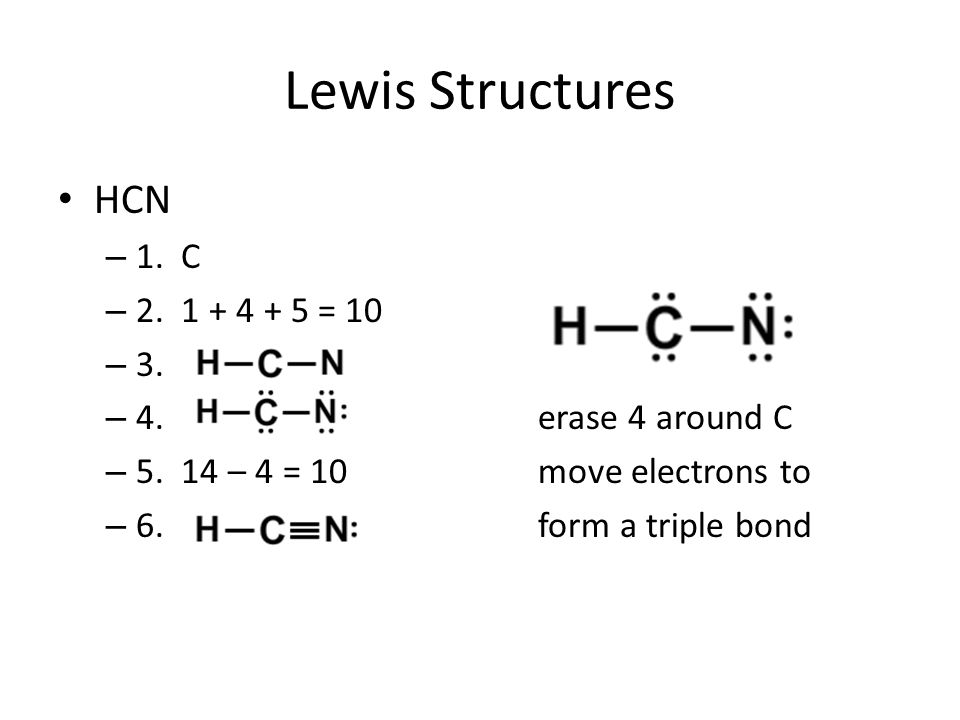
Draw the lewis structure for hcn. Draw the lewis structure for hcn. Add these electrons to give every atom an octet you nave to put a triple bond between c. A simple procedure for drawing lewis dot structures was given in a previous post entitled lewis structures and the octet rule.
It is one of the few which features a nitrogen triple bond. Draw a lewis structure for hydrogen cyanide and cyanogen. Use the properties of sodium cyanide to deduce the type of bonding that holds this compound. How is the lewis structure useful in chemistry?
- Massey Ferguson 135 Alternator Wiring Diagram
- John Deere 46 Inch Mower Deck Belt Diagram
- Bluetooth Transmitter And Receiver Circuit Diagram
For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. There are 5 steps that you need to follow when drawing the lewis structure of co (carbon monoxide). Introduction and practice problems on many molecules with answers and solutions. Lewis structure of hcn looks like.
How to draw lewis structures following the octet and duet rules. Do the same exercise for structure #2 and you find that the negative charge is on nitrogen. We then combine electrons to form covalent bonds until we come up with a lewis structure in which all of the elements. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet.
While lewis structures are useful for describing chemical bonding, they are limited in that they do not account for aromaticity, nor do they accurately. Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to. A lewis structure is a diagram that shows the covalent bonds and lone electron pairs in a molecule. The molecule hcn has one of the most fascinating lewis structures.
Clacium carbonate does not have lewis structure as whole compound because it is ion compound. Lewis structures and resonance structures. Determine the total number of valence (outer shell) electrons in the molecule or ion. Why does carbon prefer to have a triple bond with nitrogen, rather than have a double bond with nitrogen and a double bond with oxygen?
To recognize molecules that are likely to have multiple covalent bonds. To draw lewis structure is extremely easy and quick. Let us determine the lewis structures of of2 and hcn as examples in following this procedure: After determining how many valence electrons there are in.
The lewis structure (lewis dot diagram) for hcn. Lewis structures are based on the octet rule. Draw the lewis structure of hcn. Draw the skeletal structure showing how the atoms are connected using single bonds.
Put one electron pair in each bond 4. We start by writing symbols that contain the correct number of valence electrons for the atoms in the molecule. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of example 7.4. The lewis structure of a compound can be generated by trial and error.
The structure on the right is the lewis electron structure, or lewis structure, for h2o. Draw a skeleton structure put the least electronegative atom c in the middle with h and cl on either side. Diagram of a molecule using dots to represent valence electrons. Explain how you arrived at this conclusions.
Lewis's structures show each atom in the structure of the molecule using its chemical symbol. Alternatively a dot method can be used to draw the hcn lewis structure. Lines are drawn between atoms that are bonded to one another (rarely, pairs of dots are used instead of lines). As hcn has ten valence electrons for the lewis structure, now there are no electrons left.
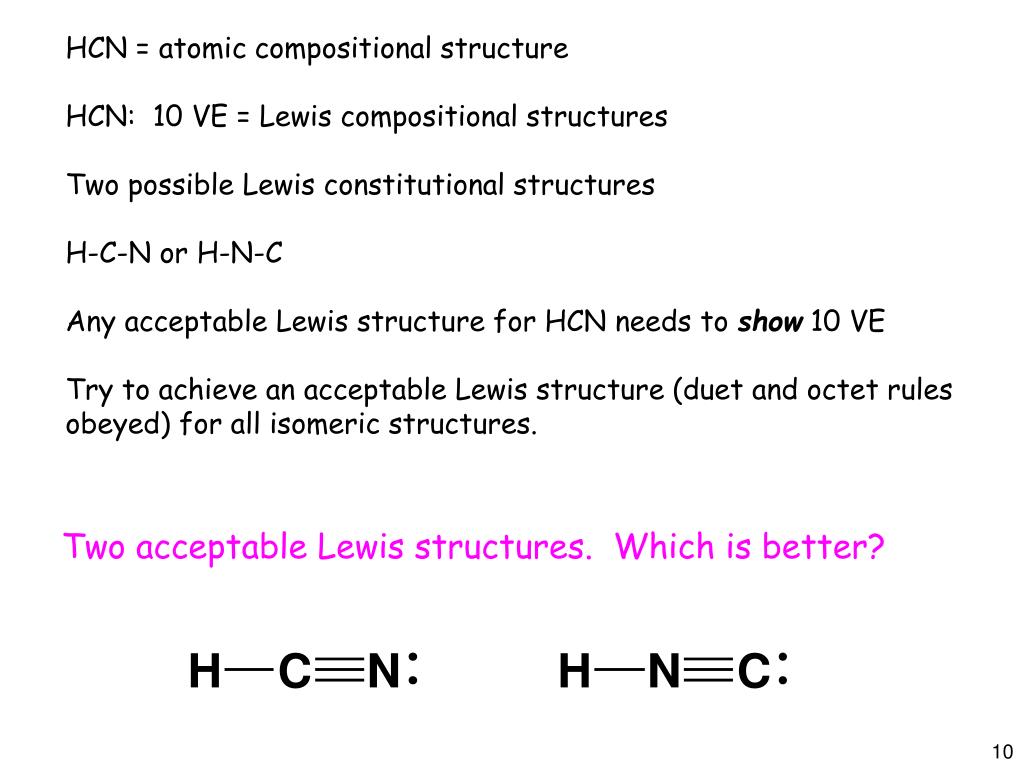
But why is structure number one preferred over structure number two? The books version is correct, because everything at the end it is the same thig altogether, but one must get the reasoning accordingly. According to the honc rule, how many covalent bonds form around hydrogen and the halogens? Let's follow the following steps to write down the lewis structure for water, methane, and carbon dioxide.
Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. Put least electronegative atom in centre 3. What is the lewis dot structure for hcn? First of all, refer to the periodic table and count the electrons by matching the columns.
Write a balance chemical equation for the reaction that occurs when sodium cyanide dissolves in water. Hcn has a linear geometry but unlike nonpolar co2 is polar due to the intrinsic electronegativity on nitrogen and the stronger force by coulomb's law pulling on the electrons. In drawing lewis structures, a single line (single bond) between two elements represents which of the following is a correct lewis structure for hydrogen cyanide, hcn? The nitrogen is having a full octet by having eight electrons in total.
Usually try to draw the most symmetrical structure with the atom of least electronegativity in the center.