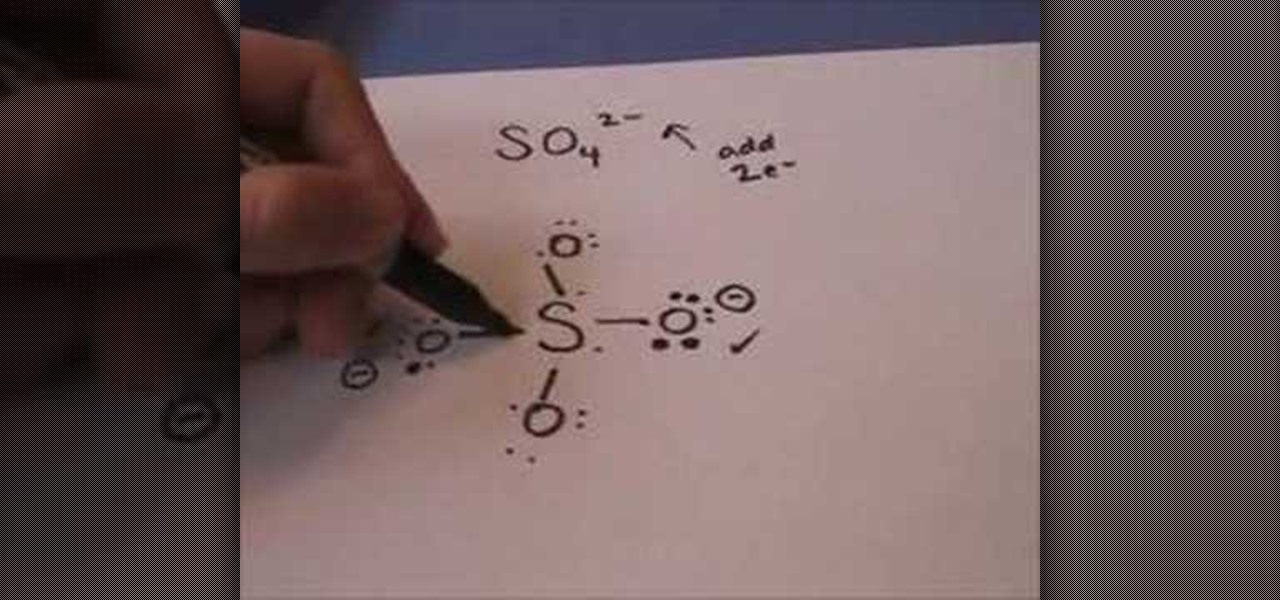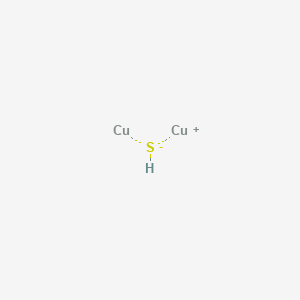Lewis Structure For Copper
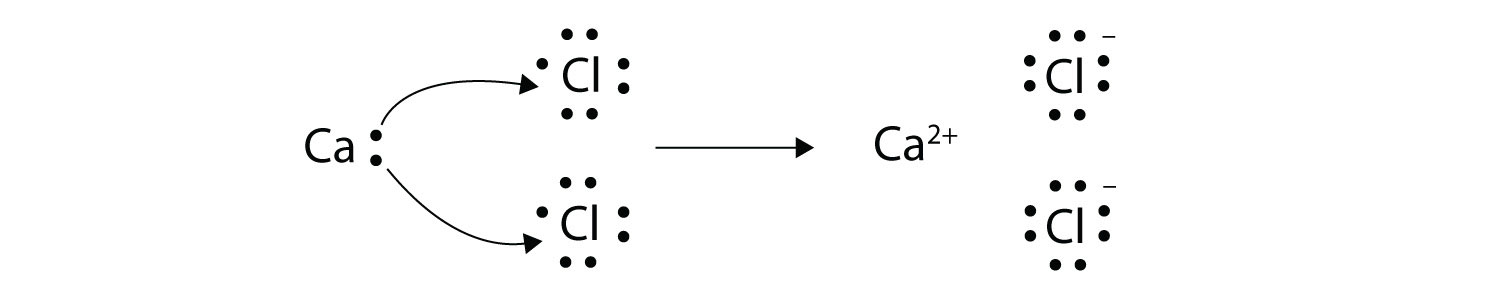
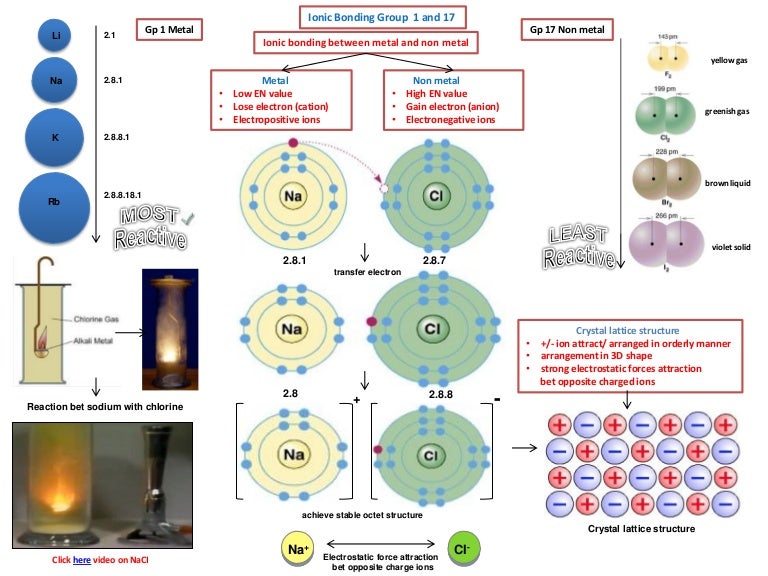
We usually use lewis dot structures to talk about covalent bonding rather than ionic bonding example:
Lewis structure for copper. Lewis, who described them in a 1916 article titled, the atom and the molecule. you can draw a lewis dot structure for any covalent molecule or coordination compound. Lewis dot structure bond length molecular geometry molecular formula molecular geometry? Why does carbon prefer to have a triple bond with nitrogen, rather than have a double bond with nitrogen and a double bond with oxygen? (1) you have two chlorine atoms.
It is yellowish red in physical appearance and when polished develops a bright metallic lustre. Sketch the atomic structure of copper and discuss why it is a good conductor and how its structure different from ge and si. It assumes that bonds and lone pairs repel each other, and will arrange themselves to be as far from each other as possible. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.
The lewis structure, or lewis dot diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The lewis structure for co has 10 valence electrons. A lewis structure shows the placement of the valence electrons around each of the atoms in a molecule. A single bond can be represented by the.
We draw lewis structures to. So the final structure would be. Cannot be described by a single lewis structure actual structure an average of the possible lewis resonance structures Grade 8 periodic table and elements.
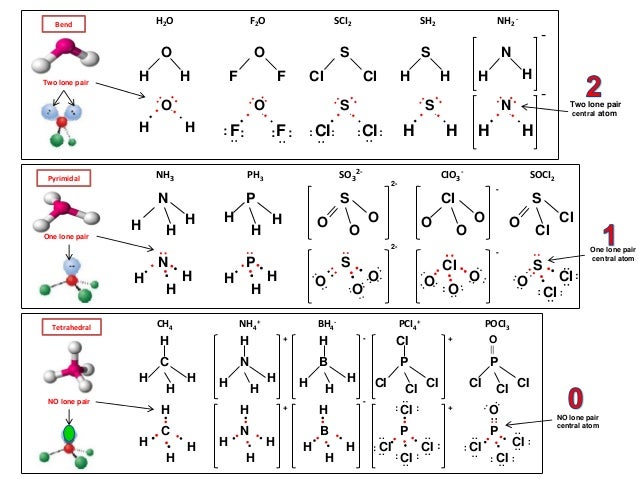
Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Most lewis structures will follow the octet rule, which states that the outer (valence) shell is stable when it has eight electrons. Copper is known to possess certain unique qualities that make it the best engineering material for bearing applications.
Carbon tetrachloride (ccl4) is a covalently. Lewis structures, also known as electron dot structures, are named after gilbert n. We start by writing symbols that contain the correct number of valence electrons for the atoms in the molecule. For the co lewis structure, calculate the total number of valence electrons for the co molecule.
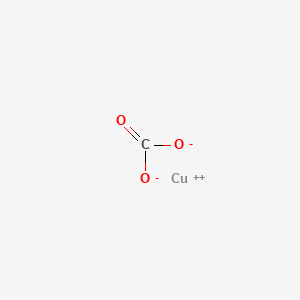
The lewis structure of a compound can be generated by trial and error. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Lewis dot structure (the 8 statements below preceded so, copper donates 2 electrons to sulfur to form an ionic species. The basic properties of copper alloys are largely influenced by the properties of copper itself.
Most lewis structures you encounter will be covalent bonds. Cu ii lewis dot dot structure for cooper copper sulfate pentahydrate dot structure electron dot structure of cuso4. < valence shell electron pair repulsion (vsepr) theory, along with lewis structures can be used to predict molecular geometry. There are many exceptions to this rule, but it should be used as a general guide for creating lewis structures.
We then combine electrons to form covalent bonds until we come up with a lewis structure in which all of the elements. Lewis dot structures (or just lewis structures) were developed around 1920 by pioneering chemist gilbert lewis, as a way of picturing chemical bonding in molecules. In drawing lewis structures for relatively small molecules and polyatomic ions, the structures tend to be more stable when they are compact and symmetrical rather than extended. Draw lewis structures depicting the bonding in simple molecules.
Copper wants to donate 2 electrons for a full valence shell (see it's column in the periodic table). The structure on the right is the lewis electron structure, or lewis structure, for h2o. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Organic chemistry lewis structures and bonding lewis dot diagram.
Thus oxygen would be left with two lone pairs of electron on it, while cl would be left with 3 pairs of electrons. Writing lewis structures with the octet rule. Lewis dot structure of atoms link. After determining how many valence electrons there are in co, place them around the central atom to complete the octets.
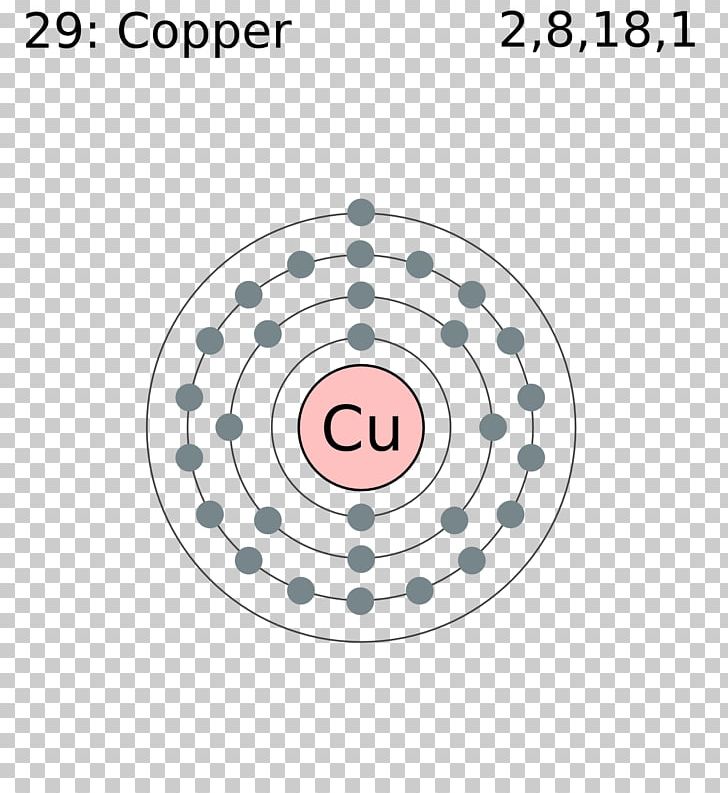
Copper has a face centred cubic crystal structure. Lewis symbols and lewis diagrams can be used to describe multiple bonds, but further information must be supplied to account for the three dimensional geometry of the resulting molecules. Do the same exercise for structure #2 and you find that the negative charge is on nitrogen. The lewis structure for li is li with one dot to the right of the element.
For more complicated molecules and molecular ions, it is helpful to follow the. What are lone pairs and how are they represented in a lewis dot diagram? Lewis structures and resonance structures. General rules for drawing lewis structures.
How is the total number of electrons represented in a lewis structure determined? For multiple single bonds, the procedure is similar that for a single bond. But why is structure number one preferred over structure number two? The lewis structure for the element copper is shown.
Discover the bonding arrangement of atoms A ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. All valence electrons of the atoms in lewis structures must be shown. Lewis structures of h2o and so2:
**when drawing lewis dot structures you usually put the atom in the center who can make the most bonds.