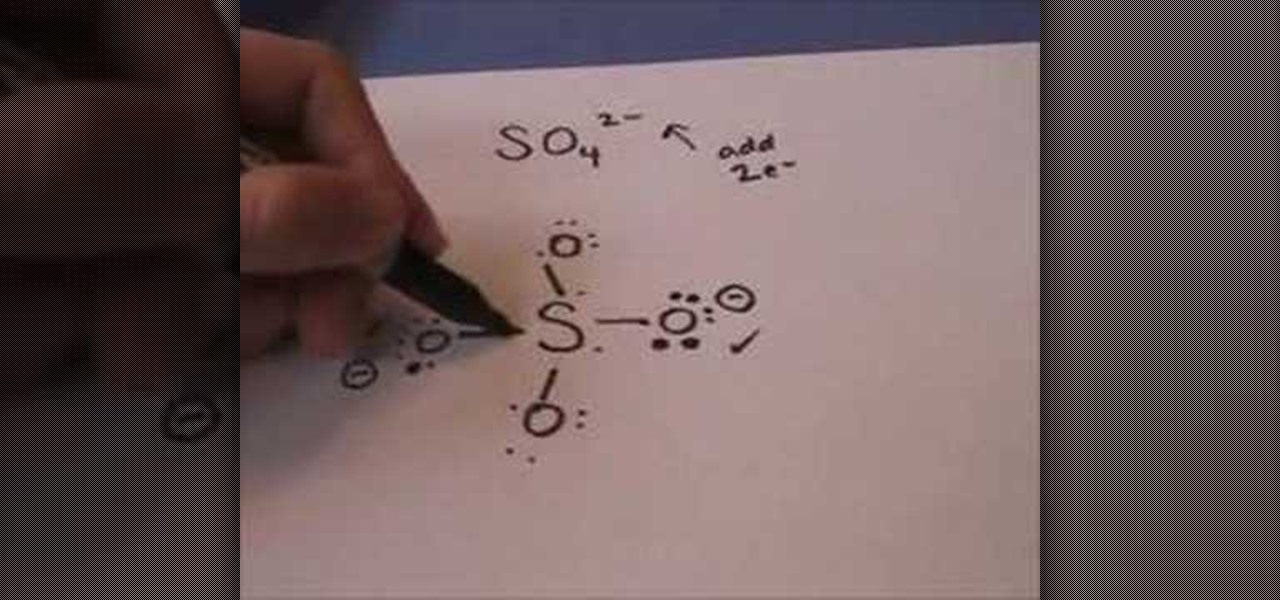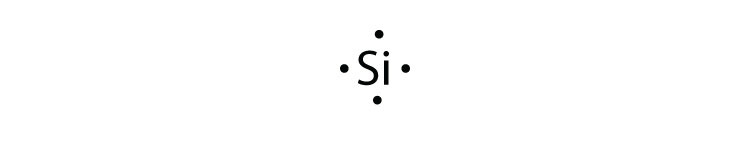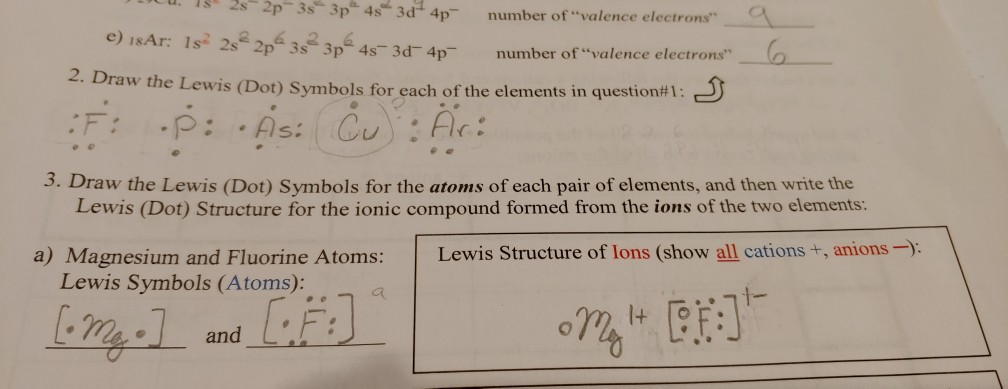Lewis Dot Structure For Copper
Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Lewis dot structure for copper. Lewis symbols of the main group elements. These steps are easy to understand and implement. 3 localized model limitations it is important to keep in mind that. How to analyze different ways to draw the dot structure for sulfur dioxide.
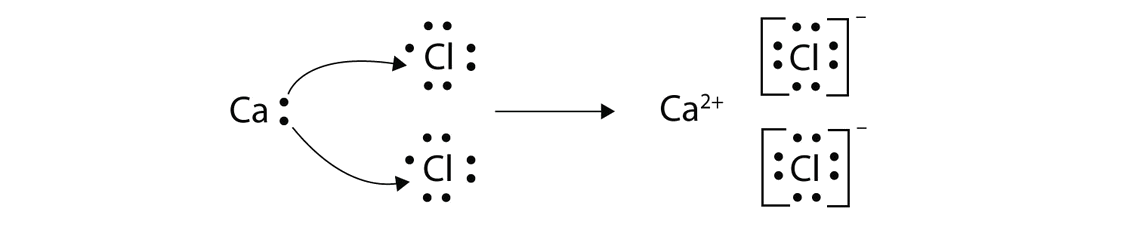
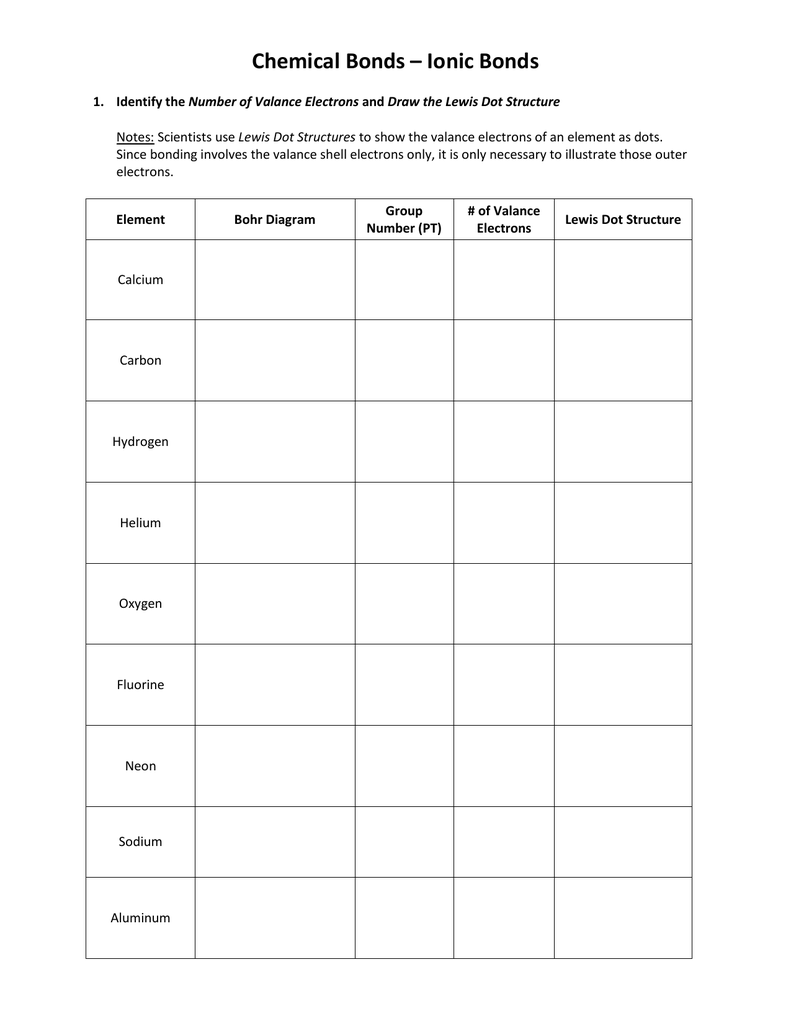
Draw the lewis dot structure for the atom and the ion. Lewis dot structures help predict molecular geometry. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping. .electrons and draw the lewis dot structure notes:
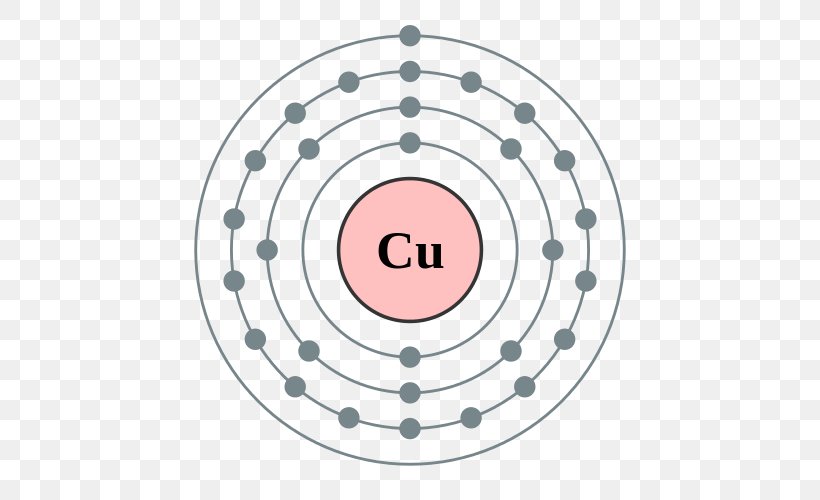
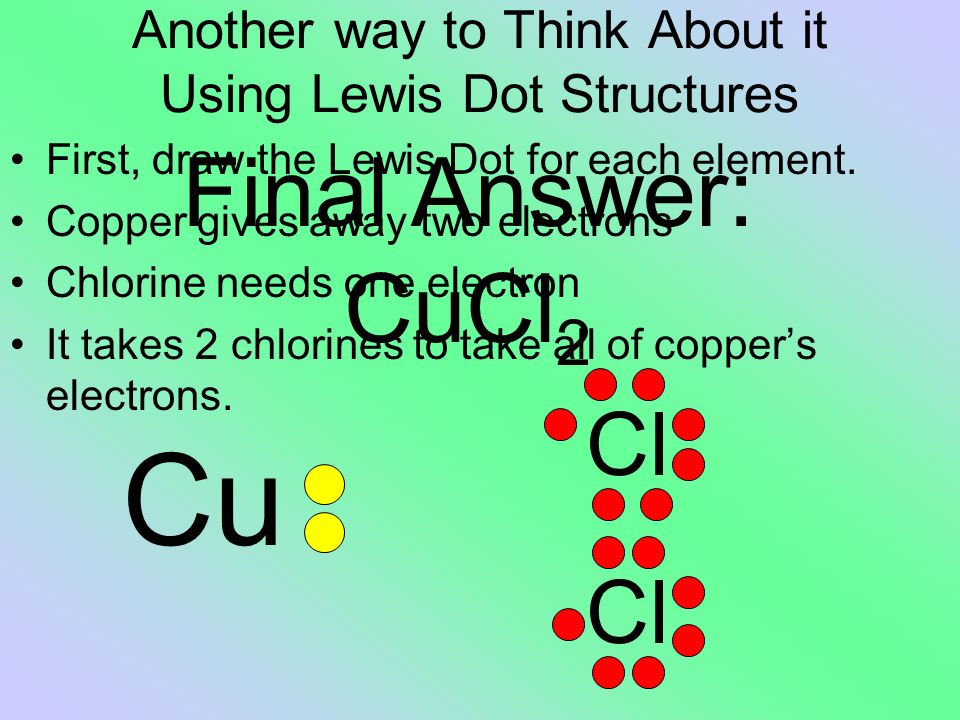
For cu(oh)2 we have an ionic compound and we need to take that into account when. Lewis structures and the octet rule. Recall the lewis structure formalism for representing lewis symbols: Copper is also an unique element that it steals an electron from its outer shell to fill in one of its inner shells.this is something you'll probably learn in high school.
- F350 Suspension Diagram
- Club Car Battery Wiring Diagram 48 Volt
- 2003 Gmc Envoy Xl Radio Wiring Diagram
Lewis dot structure of atoms link. Scientists use lewis dot structures to show the valance electrons of an element as dots. We use lewis dot structures based on valence electrons. You can create renderings of lewis dot structures with the chemdoodle web components.
2 localized bond models consider our energy diagram for h 2 bonding: The major reason why learning lewis dot structure is important is that it helps in predicting the number and type of bonds which can be formed around an atom. This is done by setting up the data and a few styles for the this function creates a molecule that represents the input molecule as a lewis dot structure using 90° angles and appropriately place lone pairs to. So the lewis structure of the diatomic chlorine molecule is shown there at the bottom.
Organic chemistry lewis structures and bonding lewis dot diagram. Lewis structure is very important in chemistry, because they are used in many important concepts of general chemistry such as chemical bonding, resonance, valence shell we can learn to make accurate lewis dot structures in 4 simple steps. The next thing i'm going to do is go through nine steps, that you can follow to draw. The valence electrons are represented by dots placed around the.
Draw two valid lewis resonance structures for the molecule ch3coo Lewis dot structure bond length molecular geometry molecular formula molecular geometry? Remember that lewis dot structures are drawn for covalent (molecular) compounds that share electrons. Gilbert newton lewis the chemist who discovered the covalent bond and created the lewis dot structure method of depicting molecules.
The lewis structure, or lewis dot diagram, shows the bonding between atoms of a molecule and any electrons that may exist. Follow these simple steps to correctly draw a. Sketch the atomic structure of copper and discuss why it is a good conductor and how its structure different from ge and si. How is the total number of electrons represented in a lewis structure determined?
When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. Lewis symbols use dots to visually represent the valence electrons of an atom. This example problem shows the steps to draw a structure where an atom violates the octet rule. Learn about an exception to the octet rule:
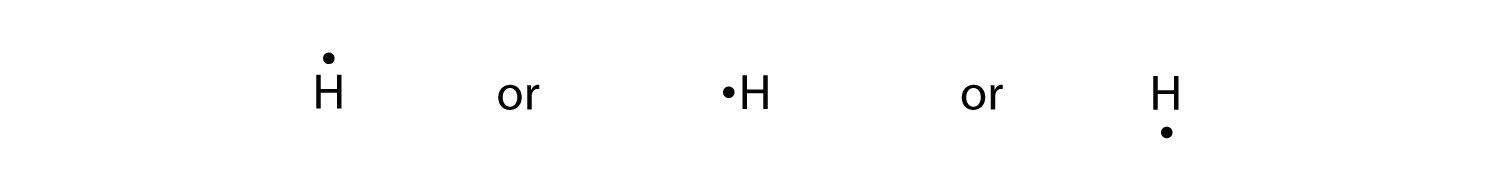
Valence electrons valence electrons are the electrons in the highest in a lewis structure, the nucleus of the element is represented by its symbol. What are lone pairs and how are they represented in a lewis dot diagram? An automatic procedure for writing canonical forms. j. In lewis dot structures each dot represents an electron.
Lewis symbols (also known as lewis dot. In chemistry, drawing lewis dot structures can be challenging, but they provide a wealth of information about the molecules they represent. Cu ii lewis dot dot structure for cooper copper sulfate pentahydrate dot structure electron dot structure of cuso4. This is a series of lectures in videos covering chemistry topics taught in high schools.
Symbols of the elements with their number of valence electrons represented as dots. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. Atoms have a lewis dot symbol with a dot for each valence electron. Name 8 potassium sulfide 9 potassium phosphide 10 beryllium fluoride cation anion 11 sr i2 12 k f 13 ca o 14 copper (ii) fluoride 15 tin (i) sulfide 16.
There is no lewis structure for sodium iodide it is an ionic bond so therefore requires an electron dot diagram. Lewis structure is basically a graphic representation of the electron distribution around an atom. 13.51, 13.53, 13.57, outline lewis dot structure basics resonance formal charge. Copper wants to donate 2 electrons for a full valence shell (see it's column in the periodic table).
Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired.