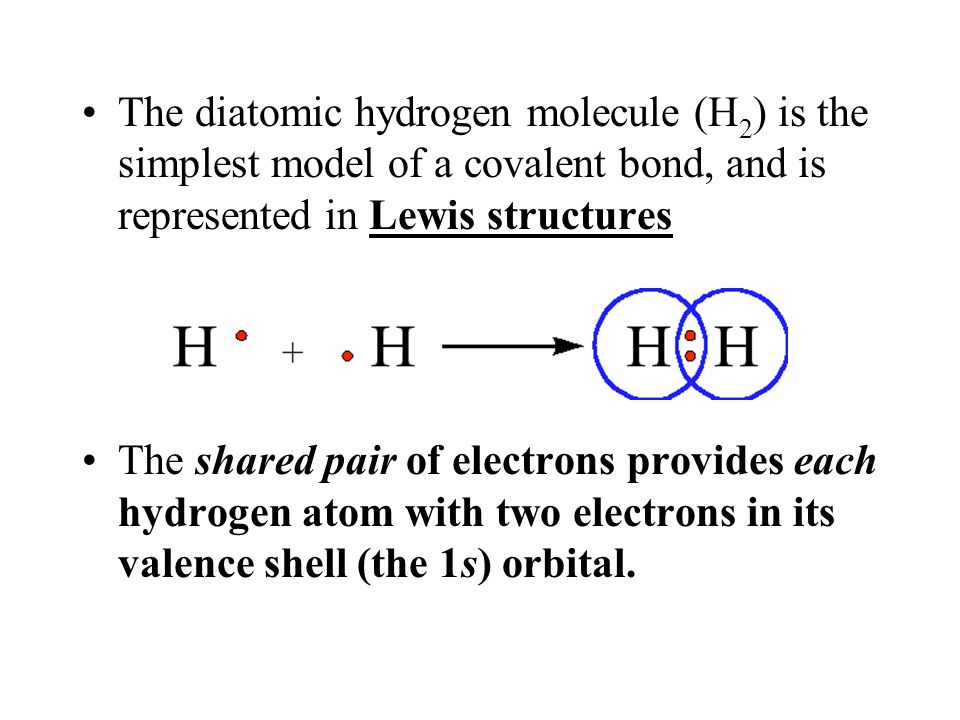
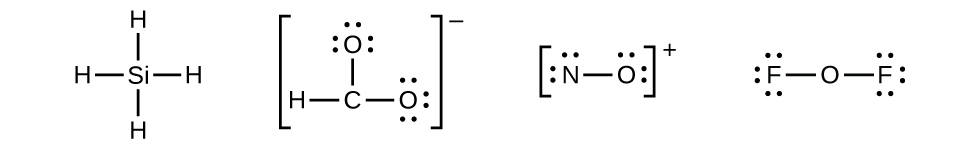Lewis Dot Structure For H2
For the h2 lewis structure, calculate the total number of valence.
Lewis dot structure for h2. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. The valence electron configurations of the constituent atoms of a covalent compound are the structure on the right is the lewis electron structure, or lewis structure, for h2o. Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. This is done by setting up the data and a few styles for the canvas.
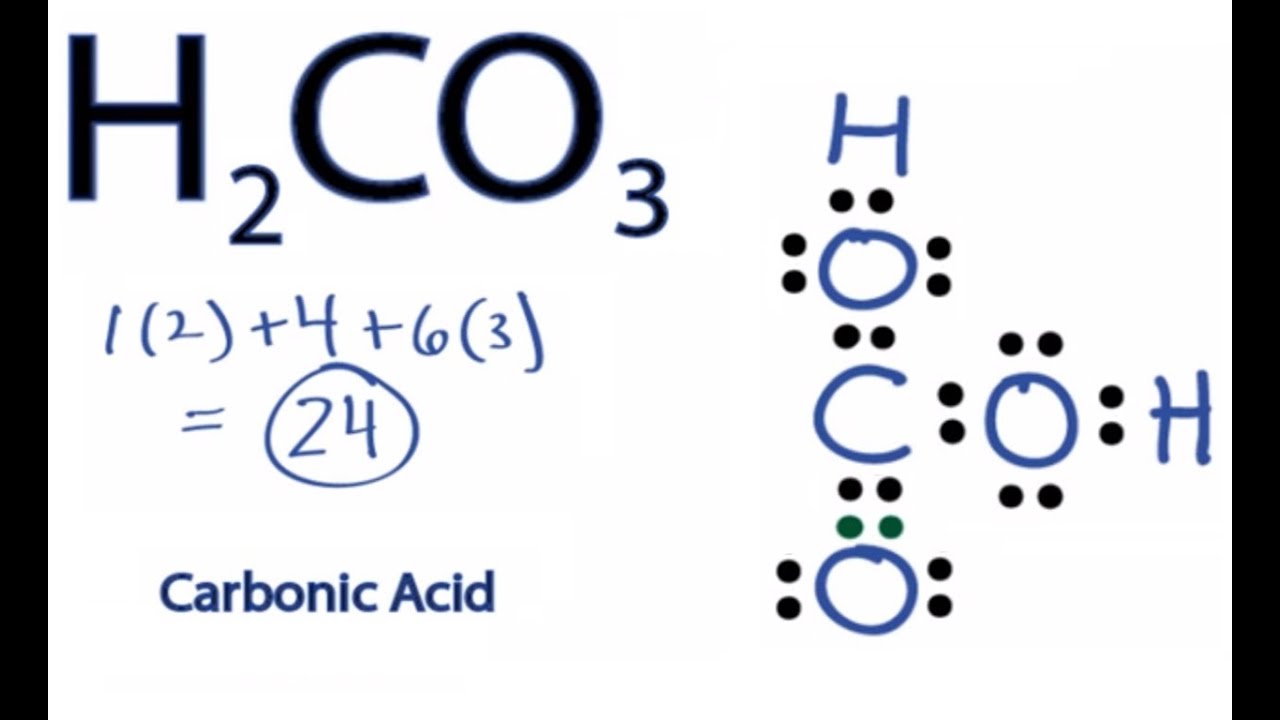
It is an ionic compound so it would not have a lewis dot structure. How can you determine the lewis dot structure of h2o2? You can create renderings of lewis dot structures with the chemdoodle web components. Lewis dot for h2o2 can be done this way.
A lewis structure is a structural representation of a molecule where dots are used to show electron position around the atoms. Person of the day g.n. Lewis structures (also known as lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping.
- 2006 Gmc Sierra 2500hd Radio Wiring Diagram
- Stihl Ts420 Parts Diagram Pdf
- Honda Gx390 Charging System Wiring Diagram
In lewis dot structures each dot represents an electron. Add up all valence electrons in the compound. Complete lewis structure by drawing atomic connectivity. A simple and general procedure for writing lewis structures is given in a previous post entitled lewis structures and the octet rule.
You have eight valence electrons in your trial structure, so it has the correct number of electrons. Lewis dot structures help predict molecular geometry. 90% of a worksheet must be completed to earn credit for that worksheet! Sulfur hexafluoride sf6 lewis dot structure.
One of the early questions asked by scientists, once the concepts of atoms and molecules had been firmly established was how are for example, salt, nacl, liquid molecules, h2o, gaseous molecules, h2 and cl2? How can i draw lewis dot structures for ionic compounds? Lewis dot structures step 1: Ad by raging bull, llc.
Draw lewis dot structures for each of the following atoms: Now you can compose your structure. The structure with both of the hydrogens and both of the fluorines bonded to the carbon allows all atoms to have the proper number of electrons. c) draw a bond (line) connecting.
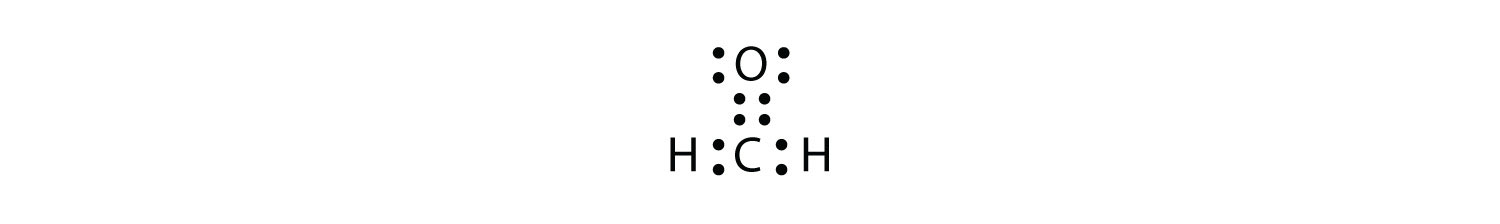
Lewis dot structure of atoms link. Each dot represents one electron. Start with each atom surrounded by its valence electrons, 5 for n, 1 for h. Lewis dot structure of h2o:
We say that there is a chemical bond between the atoms involved. Alternatively a dot method can be used to draw the h2o lewis structure. We use lewis dot structures based on valence electrons. When drawing lewis structure to be valid, each atom must have a full octet of 8 electrons (except hydrogen, which only has a duet of 2 electrons).
Write bonds in the structure and the place remaining electrons to selected atoms in the structure to give each atom an octet. Lewis structure is basically a graphic representation of the electron distribution around an atom. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. Drawing lewis dot structures (also known as lewis structures or lewis diagrams) can be confusing, particularly for a beginning chemistry student.
Using lewis dot symbols to describe covalent bonding. Lewis dot structures (or just lewis structures) were developed around 1920 by pioneering chemist gilbert lewis, as a way of picturing chemical bonding in the figure below shows how to construct the lewis structure. You can find a procedure for drawing lewis structures at this location. H2o's lewis dot structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom.
Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Well actually it can't o has 6 valence electrons and the way you used only uses 4. The trial structure is you have eight valence electrons in your trial structure, so it has the correct number of electrons. For h₂o, o must be the central atom.
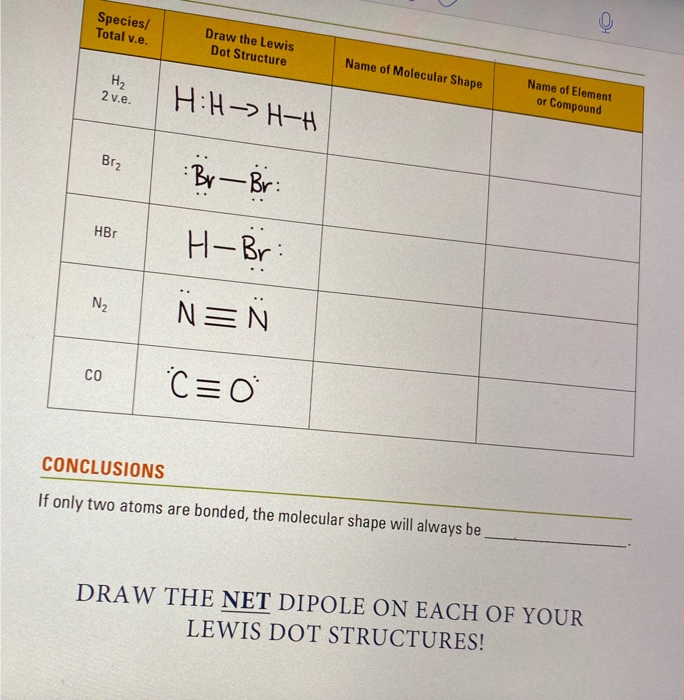
How to draw a lewis structure (octet rule exception). Lewis structure of diatomic hydrogen: Calculate the total valence electrons in the molecule. Two dots side by side represent a lone pair of electrons.
This is the process through which the h2 molecule is formed. Lewis symbols of the main group elements. How do i draw the lewis electron dot structure of cyanamide cn2h2? Lewis dot structures step 2:
For example, the calcium atom in calcium chloride, cacl2, has two valence electrons, and the chlorine atoms have seven valence electrons each. Draw the lewis dot structure for each atom of the compound to show how many valence electrons are present in each atom. H2s put h s h i. The major reason why learning lewis dot structure is important is that it helps in predicting the number and type of bonds which can be formed around an atom.
This example problem shows the steps to draw a structure where an atom violates the octet rule. This page is an index list of all of my lewis dot structure videos.