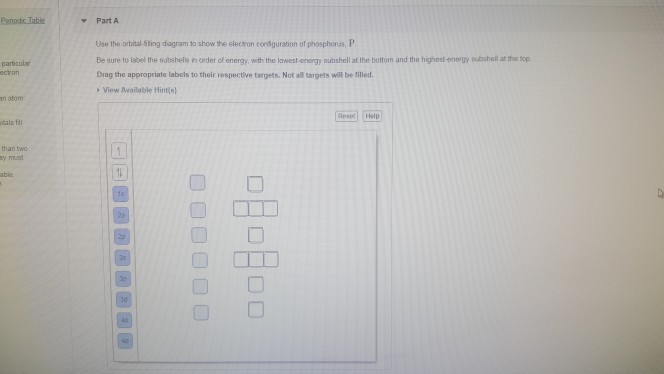
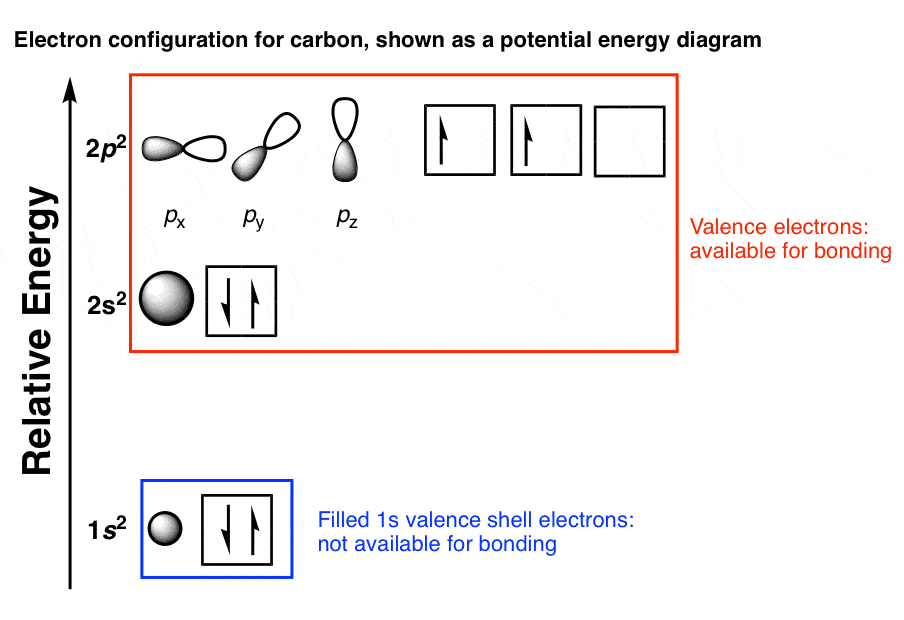
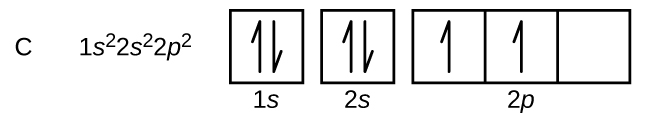
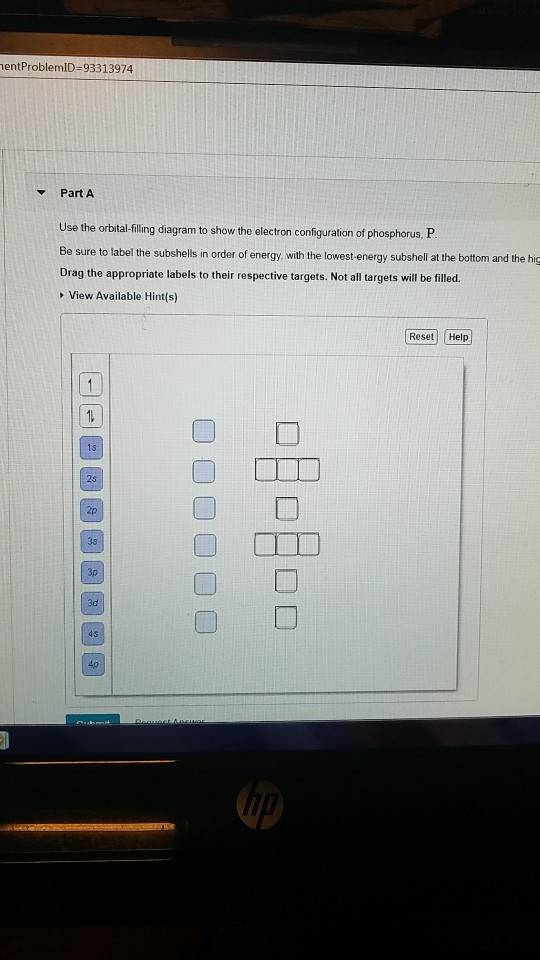
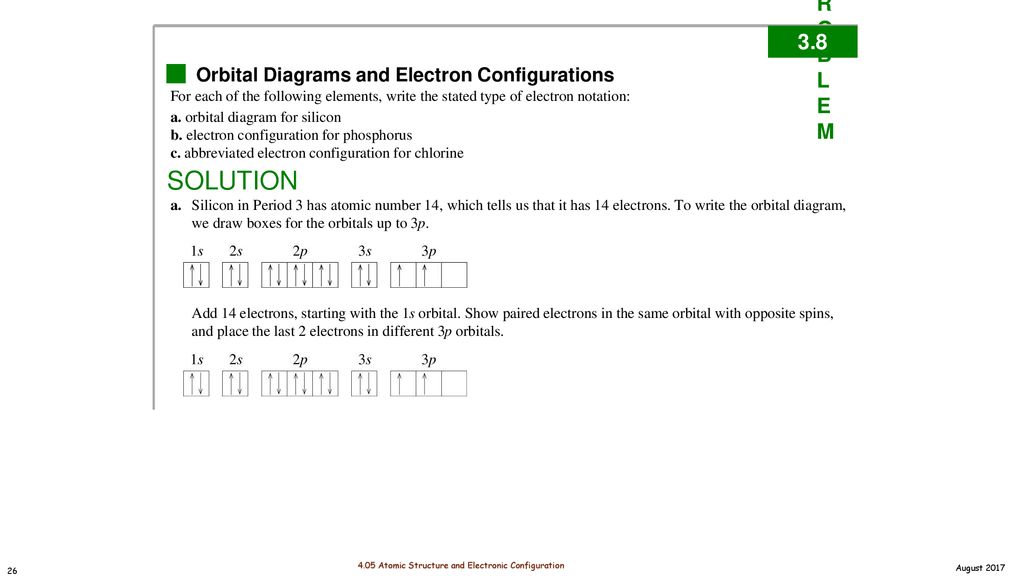
Use The Orbital Filling Diagram To Show The Electron Configuration Of Phosphorus
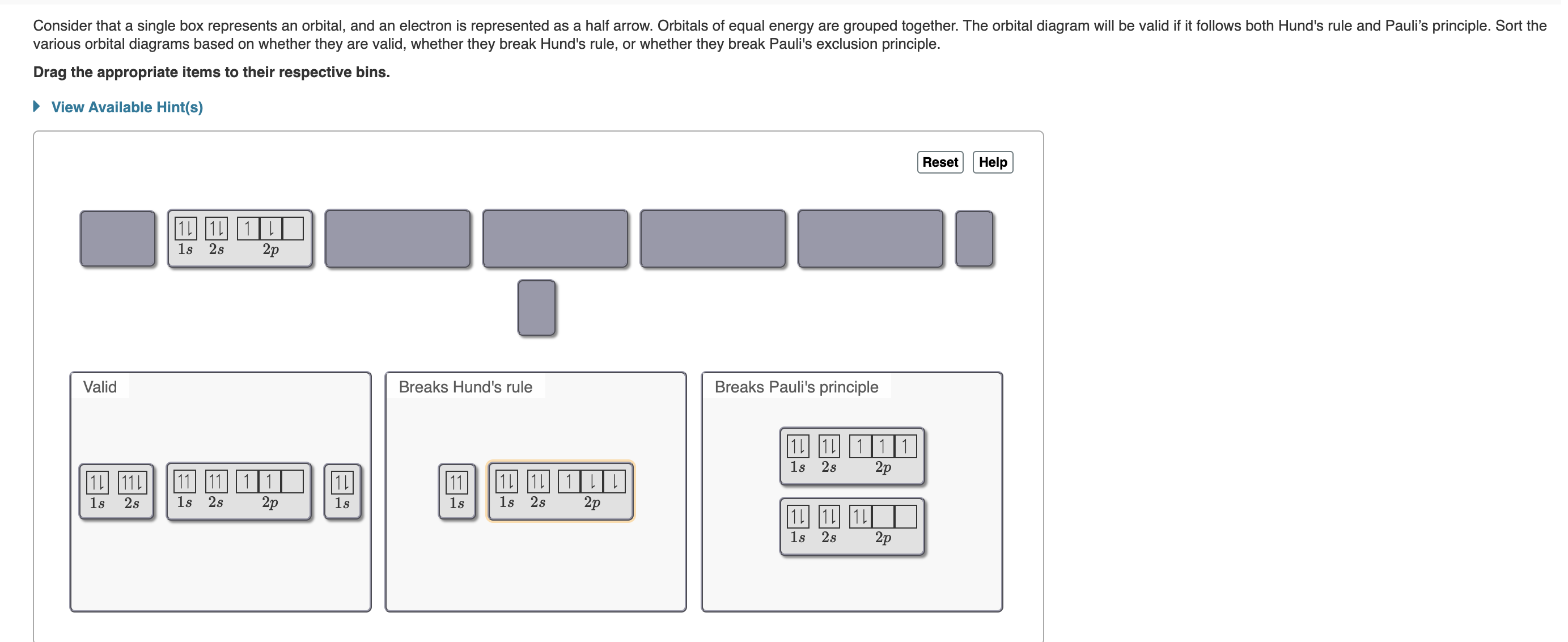
An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons.
Use the orbital filling diagram to show the electron configuration of phosphorus. Below, write the abbreviated electron configurations of the following elements:6)cobalt[ar] 4s23d77)silver[kr]. The electron configuration of an element describes how electrons are distributed in its atomic orbitals. There are 21 elements that do not follow the rule shown in that graphic, and those elements are listed below. Based on your drawing, explain why phosphorus is either the magnetic properties of a substance can be controlled by analyzing its electron configuration.
Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons. Using the periodic table on the inside cover of the text (not figure 8.10 or table 8.3), give the full and condensed electron configurations, partial orbital diagrams showing. Show an orbital diagram that would violate the pauli exclusion principle for mg (atomic number 12). Orbital diagrams orbital diagrams are pictorial descriptions of the electrons in an atom.
Drag and drop orbitals and electron counts to complete the electron configuration of iron. When electrons fill the energy levels, it fills principal write the principal energy levels and their sublevels on separate lines (as shown on the diagram). The first sublevel filled will be the using a box diagram, we show the electron configuration of nitrogen as Electron configuration shows the arrangement of electrons in an atom.
- 1998 Honda Accord Lx Stereo Wiring Diagram
- 2007 Honda Odyssey Belt Routing
- Rotary Lift Wiring Diagram
Learn how to write electronic in such cases, an abbreviated or condensed notation may be used instead of the standard notation. Where do these electrons go?. An orbital is a potential space for an electron. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
From the orbital diagram, we can write the electron configuration in an abbreviated form in which the the next element is beryllium, with z = 4 and four electrons. Thus, electronicconfiguration 1s22s22p1 corresponds to the orbital diagram Electrons possess spin and if an orbital is filled then the pair of electrons must have opposite and how to use the orbital notation to write out the full electron configuration of an element or its ion? If you need to see how electron shells are filled, click here.
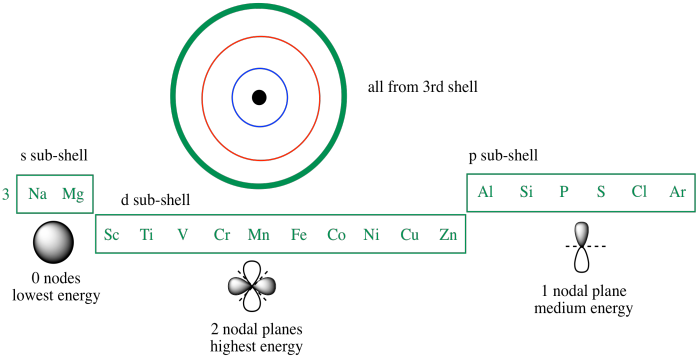
There is a specific notation that can quickly show you where use the periodic table as a visual shortcut. 3 orbital diagram another way of writing the electron configuration. We fill both the 1s and 2s orbitals to draw an orbital diagram and use it to derive the electron configuration of phosphorus, z = 15. An electron configuration shows the distribution of electrons of an atom or a molecule.
Element number of electrons electron configuration atomic radius (pm) aluminum silicon phosphorus sulfur there are several rules that scientists use to determine the electron configurations of larger atoms. You may have already noticed that the shape of the periodic table corresponds to the order of orbital sets. This orbital has 3 orientations and can therefore hold 6 total electrons. Yeung (al chemistry) quiz on atomic orbitals and electronic configuration (answer of selected questions) describe the electronic configuration of the phosphorus atom, and briefly discuss the differences between the two electrons in the 1s orbital are in the lowest energy level.
Electron configurations and orbital diagrams. Note that the calculator also shows an abbreviated way of expressing electron configuration. Electron configuration is indicated by a shorthand notation problem: Orbital diagram helium can only fill the first principal energy level.
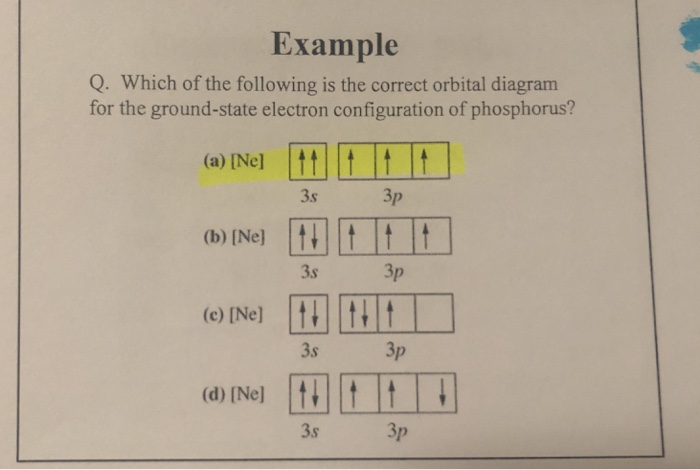
P orbital the p orbital looks like a peanut (p=peanut). In this video we'll use the electron configuration chart to help us write the notation for phosphorus. In the electron configurations of main group elements lesson and manner in which the orbitals in a p sublevel are filled, we have to introduce a symbolic notation that scientists use to show orbital filling. Notice how the two 2p electrons in the orbital diagram on the left are in separate orbitals.
Use the buttons at the top of the tool to write the electron configuration of oxygen. An element's electron configuration can be represented using energy level diagrams, or aufbau diagrams. The arrangement of electrons in the shells and orbitals is called the electronic configuration or. This breaks pauli's exclusion principle because no 2 electrons can have the same 4 quantum numbers.
In the abbreviated notation, the sequence of completely filled. In order to write the phosphorus electron configuration we first need to know the number of since 1s can only hold two electrons the next 2 electrons for phosphorous go in the 2s orbital. 60 154 просмотра 60 тыс. The first row is the ground state electron configuration and orbital diagram for the following.
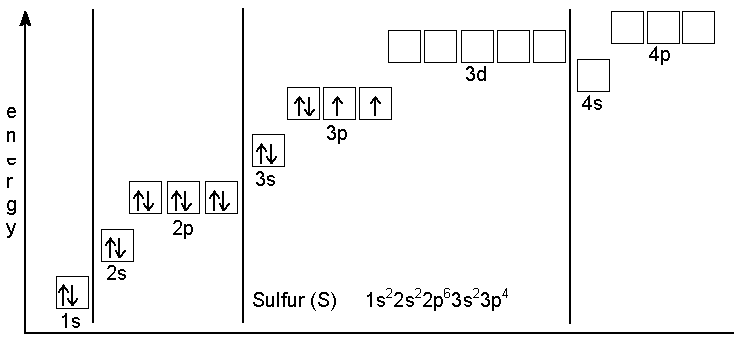
How to depict the electronic configuration of atoms using orbital diagrams. You don't need to include the orbital box diagram as part of your answer. The table below shows the electron configurations of the elements in the second period. Orbitals are filled from lowest energy to highest energy orbital diagrams • shows the distribution of electrons within orbitals • circles/lines/boxes are used to represent the different orbitals • arrows are used to.
The every orbital in a subshell is singly occupied with one electron before any one electronic configuration of simple phosphorus (p) atom filling method is shown below Electrons fill orbitals starting at the lowest available energy state before filling higher states. If it has unpaired electrons in its outer orbital, at that point the. Using standard notation, the electron configuration of fluorine is 1s22s22p5.
Draw an orbital diagram to show the arrangement of the hydrogen electrons. The electronic configuration is the distribution of electrons of a given molecule or hund's rule: