Copper Lewis Structure
Grade 8 periodic table and elements.
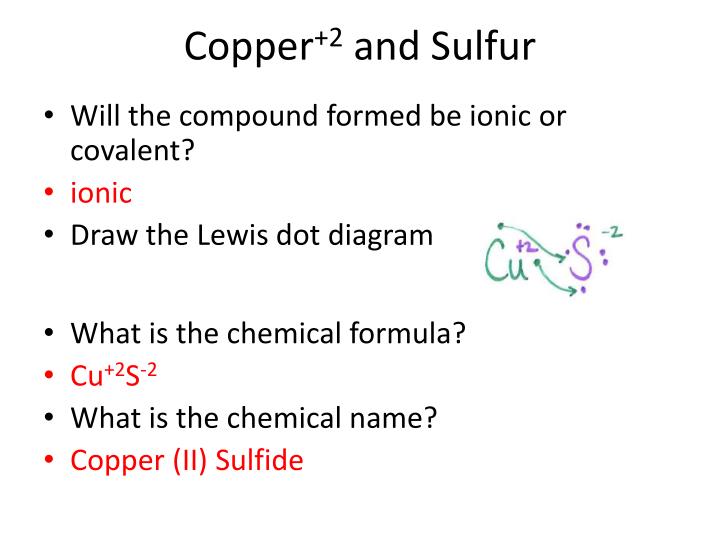
Copper lewis structure. Cu ii lewis dot dot structure for cooper copper sulfate pentahydrate dot structure electron dot structure of cuso4. The lewis structure for the element copper is shown. Lewis structures show how the electrons are arranged in the molecule. So2 has three, and therefore is trigonal planar.
For the co lewis structure you'll need a triple bond between the carbon and oxygen atoms in order to satisfy the octets of each atom while. We draw lewis structures to. Copper is a chemical element with the symbol cu (from latin: Hydrogen often is bonded to carbon, nitrogen or oxygen.
The lewis structure for co has 10 valence electrons. Lewis dot structure (the 8 statements below preceded by dashes and the lewis dot periodic copper wants to donate 2 electrons for a full valence shell (see it's column in the periodic table). Lewis dot structure bond length molecular geometry molecular formula molecular geometry? The diagram opposite shows the lewis structure for the water molecule, h2o, and the ethene molecule, c2h4.
- 2002 Ford Taurus Coolant System Diagram
- Adjust Sump Pump Float
- 1985 Chevy Truck Steering Column Diagram
Organic chemistry lewis structures and bonding lewis dot diagram. Actual structure an average of the possible lewis resonance structures. A lewis structure shows the placement of the valence electrons around each of the atoms in a molecule. This is how the lewis dot structure looks like for silicone.
It is yellowish red in physical appearance and when polished develops a bright metallic. (a) the copper(i) ion (b) the copper(ii) ion (c) the manganese(ii) ion (d) the lead(iv) ion. The lewis structure is used to represent the covalent bonding of a molecule or ion. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity.
Lewis structures are a useful way to summarize certain information about bonding and may. How is the total number of electrons represented in a lewis structure determined? Modern day structure of the atom. They are always at the ends of a sequence of atoms.
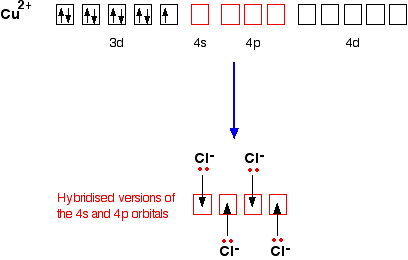
Sketch the atomic structure of copper and discuss why it is a good conductor and how its structure different. Cuprum) and atomic number 29. Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. Covalent bonds are a type of chemical bonding formed by the sharing of electrons in the valence shells of the atoms.
They are important because they allow us to predict the shapes of molecules, the types and. Lewis dot structures (or just lewis structures) were developed around 1920 by pioneering chemist gilbert lewis, as a way of picturing chemical bonding in molecules. You can draw a lewis dot structure for any covalent molecule or coordination compound. Hydrogen atoms form only single bonds;
In the example lewis structures drawn above, h2o has four bonds/lone pairs around it and therefore adopts a tetrahedral geometry. Copper has a face centred cubic crystal structure. Also an alien atom in one of the interstitials in a structure. Today's atom is a spherical existence formulated from a copper ii sulfate pentahydrate is not a molecule, strictly speaking, but you will hear the term molecular.
For cu(no3)2 we have an ionic compound and we need to take that into account. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. What is lewis dot structure of sodium sulphate?