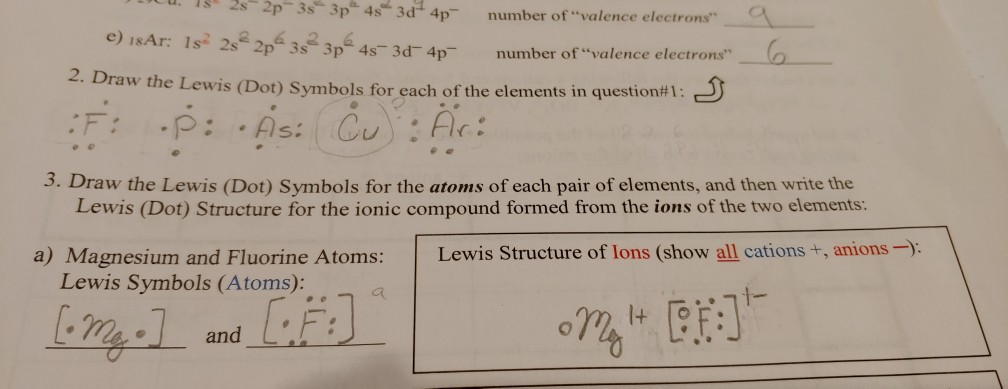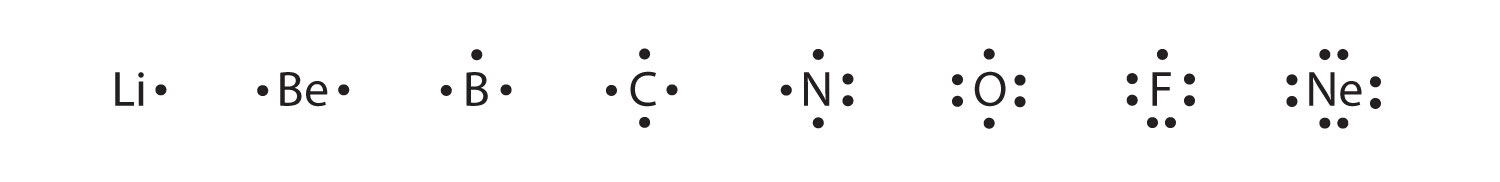Copper Lewis Dot Structure
The lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line.
Copper lewis dot structure. Remember that lewis dot structures are drawn for covalent (molecular) compounds that share electrons. In lewis dot structures each dot represents an electron. Lewis dot structure for copper. It is an ionic compound so it would not have a lewis dot structure.
However, these structures are helpful in understanding the bonding and valence electron. Lewis dot structure bond length molecular geometry molecular formula molecular geometry? Lewis structure is basically a graphic representation of the electron distribution around an atom. Two dots side by side represent a lone pair of electrons.
Each dot represents one electron. Gilbert newton lewis the chemist who discovered the covalent bond and created the lewis dot structure method of depicting molecules. Lewis dot structures (or just lewis structures) were developed around 1920 by pioneering chemist gilbert lewis, as a way of picturing chemical bonding in molecules. How is the total number of electrons represented in a lewis structure determined?
- 2008 Mercury Mariner Radio Wiring Diagram
- 2002 Ford Explorer Vacuum Diagram
- 2007 Hyundai Accent Stereo Wiring Diagram
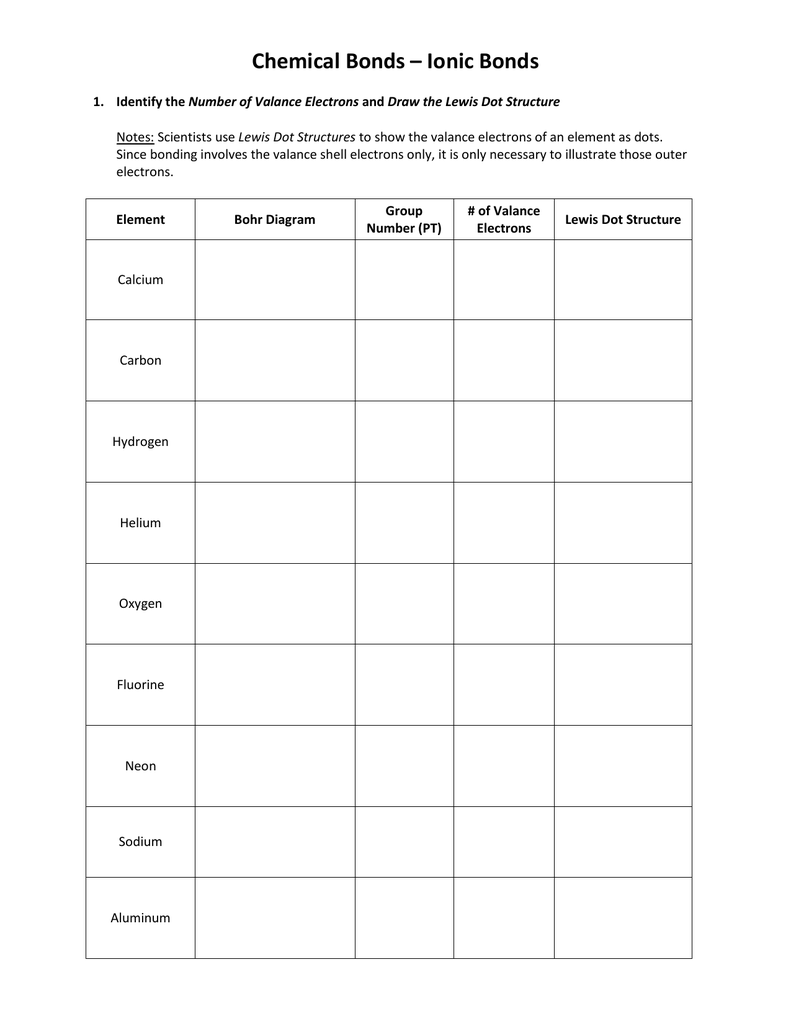
Name 8 potassium sulfide 9 potassium phosphide 10 beryllium fluoride cation anion 11 sr i2 12 k f 13 ca o 14 copper (ii) fluoride 15 tin (i) sulfide 16. This is a series of lectures in videos covering chemistry topics taught in high schools. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Symbols of the elements with their number of valence electrons represented as dots.
Copper wants to donate 2 electrons for a full valence shell (see it's column in the periodic table). .electrons and draw the lewis dot structure notes: Follow these simple steps to correctly draw a. Lewis structures and the octet rule.
Predicting compound structures and properties using a simple theory. Discover the bonding arrangement of atoms Lewis symbols of the main group elements. Scientists use lewis dot structures to show the valance electrons of an element as dots.
These steps are easy to understand and implement. So they indicates bond pairs as number of bond. The major reason why learning lewis dot structure is important is that it helps in predicting the number and type of bonds which can be formed around an atom. In chemistry, drawing lewis dot structures can be challenging, but they provide a wealth of information about the molecules they represent.
Start studying naming lewis dot structures. Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. So the lewis structure of the diatomic chlorine molecule is shown there at the bottom. Lewis dot structures help predict molecular geometry.
For cu(no3)2 we have an ionic compound and we need to take that into account. The next thing i'm going to do is go through nine steps, that you can follow to draw. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping.
Ocl− is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool. We draw lewis structures to. But i have copper, and i cant find the lewis dot diagram for that anywhere! Recall the lewis structure formalism for representing valance electrons.
Drawing lewis dot structures (also known as lewis structures or lewis diagrams) can be confusing, particularly for a beginning chemistry student. Lewis dot structure of atoms link. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. The valence electrons are represented by dots placed around the.
Organic chemistry lewis structures and bonding lewis dot diagram. Lewis symbols (also known as lewis dot diagrams or electron dot diagrams) are diagrams that represent. Sketch the atomic structure of copper and discuss why it is a good conductor and how its structure different from ge and si. An automatic procedure for writing canonical forms. j.
How lewis dot structure hel. Lewis dot represent lone pairs and bond pairs of electron. Lewis structures, part 1 of 3 learn a simple process for drawing correct lewis diagrams, and practice drawing lewis structures that obey the octet rule. Accordingly, these are called lewis dot structures for atoms.
This example problem shows the steps to draw a structure where an atom violates the octet rule. Copper bonded grounding clamps and fittings. Draw the lewis dot structure for the atom and the ion. What are lone pairs and how are they represented in a lewis dot diagram?
One of the early questions asked by scientists, once the concepts of atoms and molecules had been firmly established was how are atoms bonded? we've developed many theories over the years in attempts to explain the bonding between atoms in various substances. Valence electrons valence electrons are the electrons in the highest in a lewis structure, the nucleus of the element is represented by its symbol. Since you can only have 8 dots, 2 on. Learn vocabulary, terms and more with flashcards, games and other study tools.