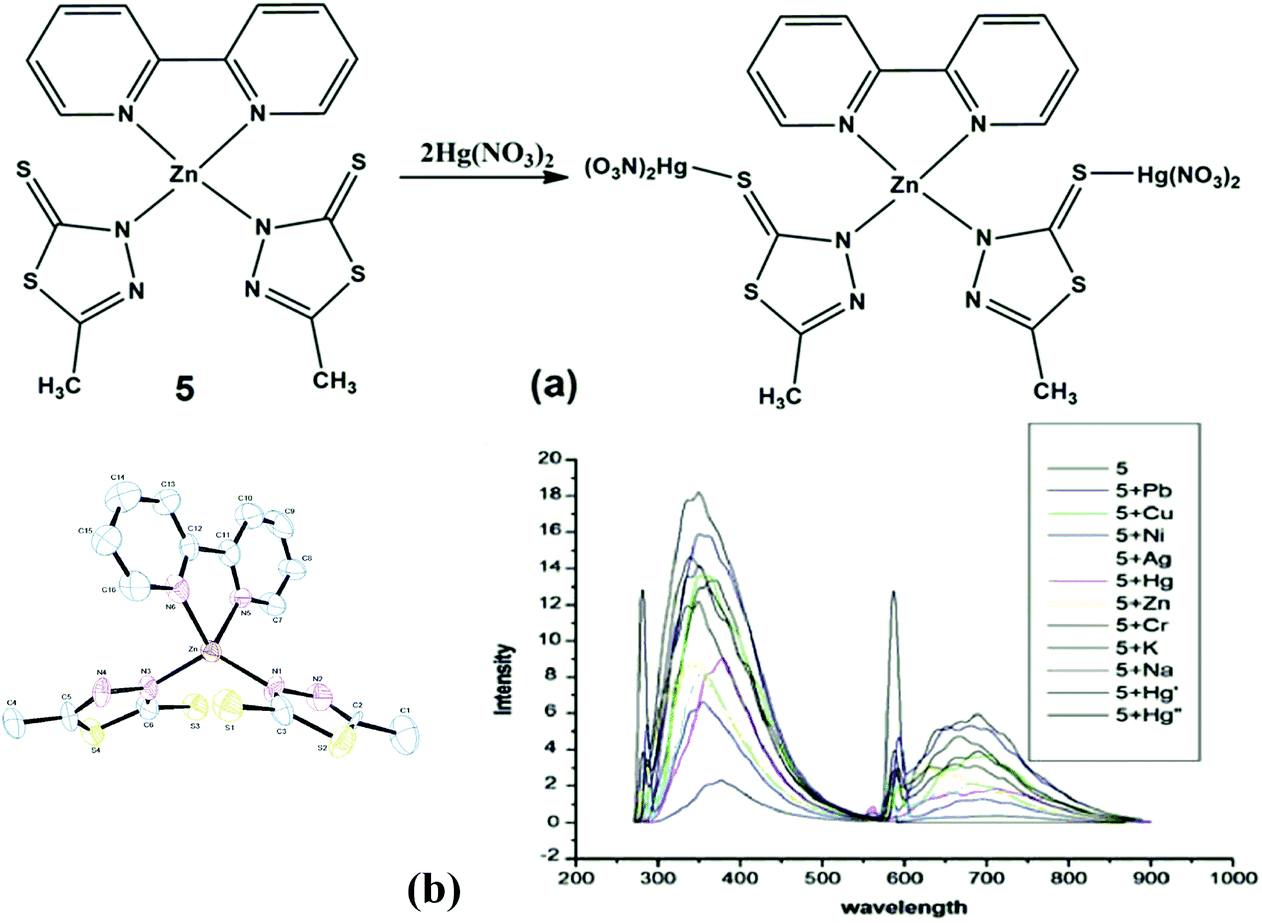
Choose The Valence Orbital Diagram That Represents The Ground State Of Zn
For main group elements, the valence electrons are the outer electrons.
Choose the valence orbital diagram that represents the ground state of zn. Lowest available energy) to some other so any electron configuration in which the last electron (again, the valence electron) is in a higher energy orbital, this element is said to be in an excited state. Three 4p boxes are completely filled. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. An element that has the valence electron configuration 5s25p5 belongs to which period and group?
An orbital that penetrates into the region occupied by core electrons is less shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy. Please join studymode to read the full document. Which of the following electron configurations represents the ground state of an element? Tags electron, valence, electron affinity, periodic table, best lewis structure.
Draw an orbital diagram for al. A) an orbital that penetrates into the region occupied by core electrons is less shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy. They are in the same group (family) and all have the same number of valence electrons. A stable electron configuration has 8 valence electrons and a full octet, meaning that their would be a happy atom and it would be non reactive.
- 96 Honda Accord Interior Fuse Box Diagram
- 5 Wire Wideband O2 Sensor Wiring Diagram
- Dodge Nitro Belt Diagram
1)choose the valence orbital diagram that represents professor hierl. Orbital diagrams orbital diagrams are pictorial descriptions of the electrons in an atom. Partial orbital diagrams and condensed configurations. Choose the valence orbital diagram that represents the ground state of zn.
An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Choose the valence orbital diagram that represents the ground state of sr2+. Valence electrons valence electrons are the outer electrons that are involved in bonding. The partial orbital diagram below where n could be any valid quantum number.
Ground state refers to the lowest energy state regardless of whether it is an atom or an ion. The ground state electron configuration of carbon, which has a total of six electrons. The symbol represents the nucleus and inner (core) electrons each dot represents a valence electron (8 maximum) follow hund's rule x. 3p 3s 3p 4p d.
Which of the following represents the lewis structure for ca2+. The number of unpaired electrons present in ground state can be determined by the study of the ground state electronic configurations of the elements. 3p 3s 3p 4p d. The configuration is determined by applying the rules of the aufbau principle.
This video provides 3 example. Choose the orbital diagram that represents the ground state of in+3.? 3) choose the valence orbital diagram that represents the ground state of zn. The orbital diagram in which 'aufbau principle' is violated, is represented by the option (b).
Explore the bohr model and atomic orbitals. Choose the ground state electron configuration for zn^2+. You are considering ions as being excited states and that is not true. In this context, n represents the principal quantum number and ℓ represents the azimuthal quantum number.
This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms) from an element or valence. Smaller nuclear charge lowers energy, more electrons in an orbital increases energy. You saw how the number and type of valence electrons are important in determining the chemical properties of a particular element. Learn how to use an element's position on the periodic table to predict its properties, electron configuration, and reactivity.
An orbital diagram helps to determine the electron configuration of an element. Electron configuration orbital diagram for each orbital containing electrons in the ground state: Orbital is an antibonding molecular orbital (a molecular orbital that forms when atomic orbitals or orbital lobes of opposite sign interact to give decreased electron probability between the nuclei due to destructive reinforcement of the wave functions). In the electron configurations of main group elements lesson, you learned a little bit about valence electrons.
An electron that exists in the orbital of an atom singly and not as a part of an electron pair is known as an unpaired electron. This comic is the third of five consecutive comics published in the week before and during the solar eclipse occurring on monday, august 21, 2017 which was visible as a total solar eclipse within a band across the contiguous united states from west to east and visible as a partial eclipse across the. 29) give the set of four quantum numbers that represent the electron lost to form the k ion from the k answer: Choose the orbital diagram that represents the ground state of n.
4 lewis (electron) dot diagrams are… a way of showing & keeping track of valence electrons. D 14) choose the valence orbital diagram that represents the ground state of se2 ⁻. Choose the valence orbital diagram that represents the ground state of zn. Choose the valence orbital diagram that represents the ground state of se2⁻.
One electron can jump from a lower to a higher energy orbital, thus creating unpaired electrons. A partial orbital diagram shows only the highest energy sublevels being filled. Orbitals are represented by boxes 1.