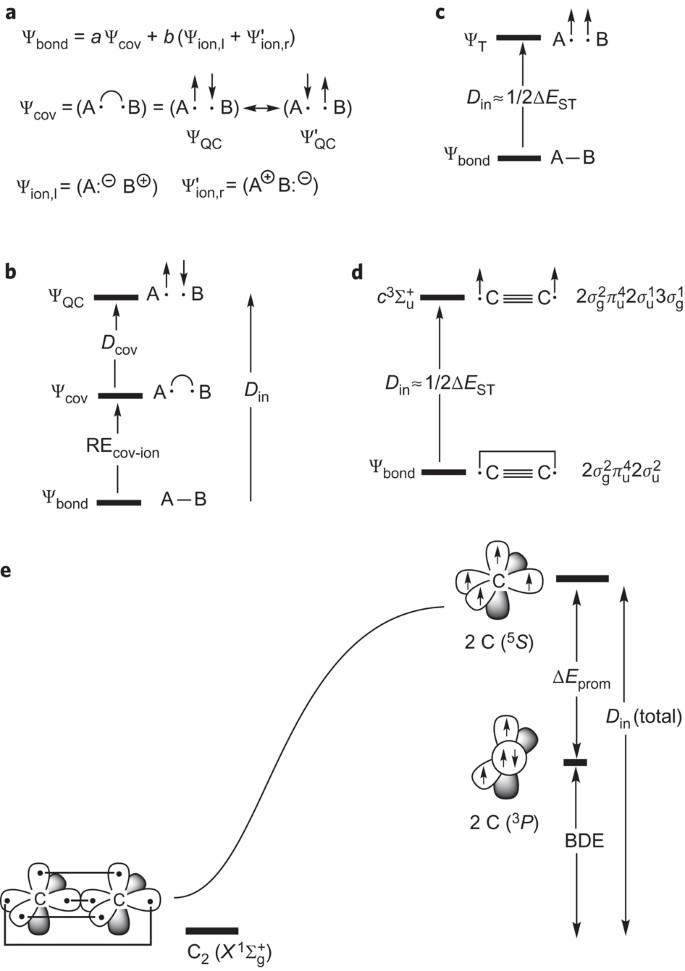
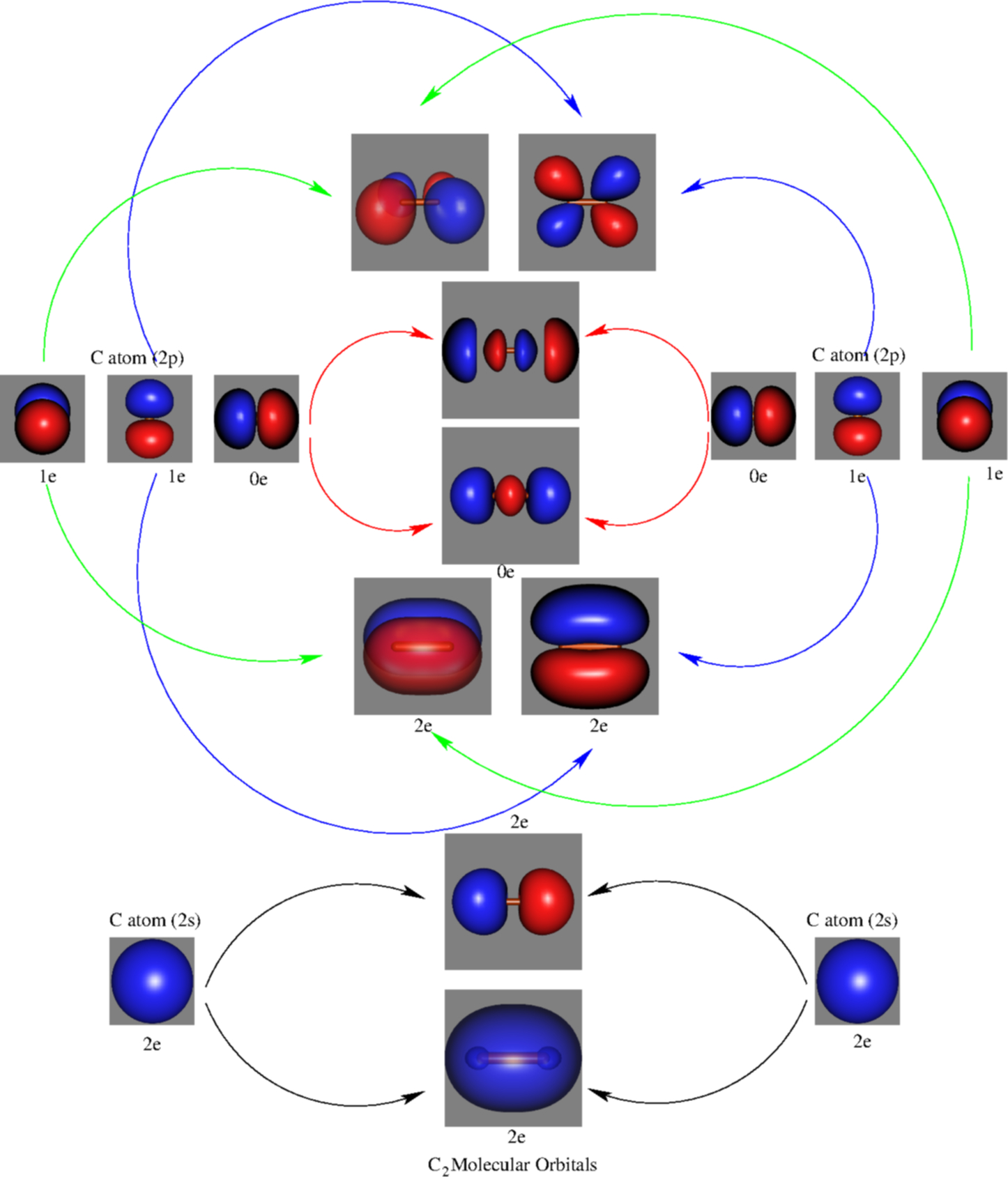
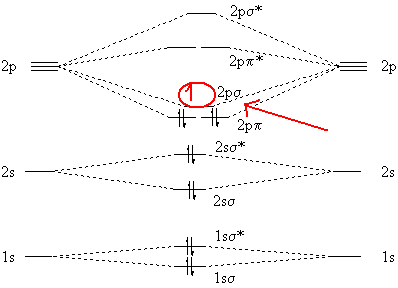
C2 2 Molecular Orbital Diagram
A molecule in which all the electrons are paired, is called diamagnetic.
C2 2 molecular orbital diagram. The problem provides you with the mo diagram for the #c_2# molecule, so all you really have to do here is add an electron to that diagram. Fill from the bottom up, with 8 electrons total. This article explains how to create molecular orbital diagrams in latex by means of the package modiagram. One atom of nitrogen has 7 electrons so a n2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bon.
The molecular orbital energy diagram predicts that he2 will not be a stable molecule, since it has equal numbers of bonding and antibonding eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Valence bond theory, hybrid orbitals, and molecular orbital theory. For example, homonuclear diatomic molecules of second row elements like li2, be2, b2 , c2, n2 , the σ. According to molecular orbital theory individual atoms combine to form molecular orbitals, as the electrons of an atom are present in various atomic orbitals and are associated with several nuclei.
Here we have a molecular orbital diagram for the co molecule. N2+ has a weaker longer bond than n2, but o2+ has a stronger, shorter bond than o2. Number of electrons in c2 molecule = 12. Part a complete the mo energy diagram for the n2+ ion by dragging the electrons electron.
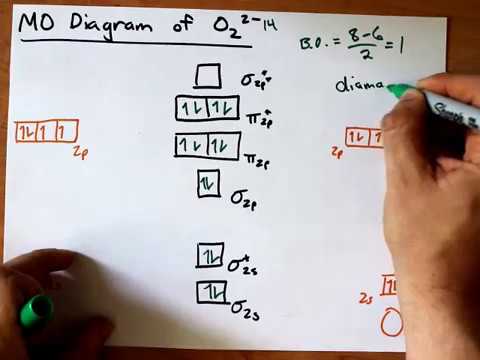
The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. The molecular orbital diagram for c2^2 Bonding order is 2, and it is diamagnetic. Molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms, that is, in terms of orbitals, orbital diagrams, and electron configurations.
Thus molecular orbital theory correctly predicts that h2 is a stable molecule. Construct a molecular orbital diagram for the hydrogen molecule ion h2+ by using. Mo energy diagram for o 2. This is the molecular orbital diagram for the homonuclear diatomic be2+, showing the molecular orbitals of the valence shell only.
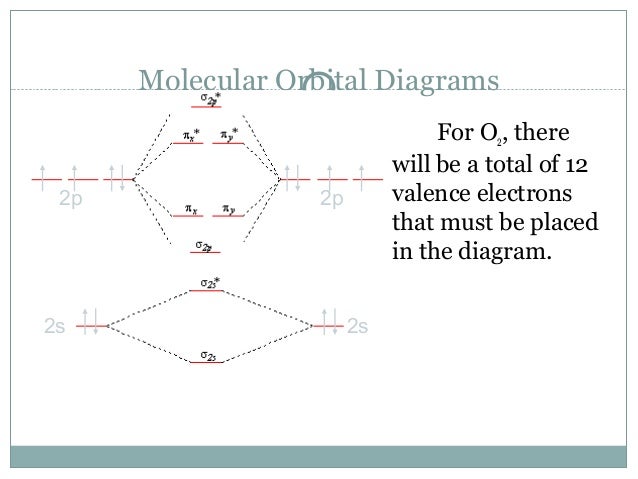
Molecular orbital theory, bonding & antibonding mo, bond order, homonuclear diatomic molecules. Molecular orbital energy diagrams for (a) h 2 showing both electrons in the bonding sigma mo; Define the axial system and find all of the symmetry operations on the molecule 3. The molecular orbital diagram for an o2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p experiments have shown that o2 and f2 are best described by the model in the figure above, but b2, c2, and n2 are best.
A mo diagram forming a mo diagram 1. Identify the symmetries and energy ordering are determined by examining the orbital phase pattern. Number of electrons in c2 molecule = 12. Fill from the bottom up, with 8 electrons total.
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Dihydrogen and its ion h2+. Start studying molecular orbital theory. Determine the molecular shape and identify the point group of the molecule 2.
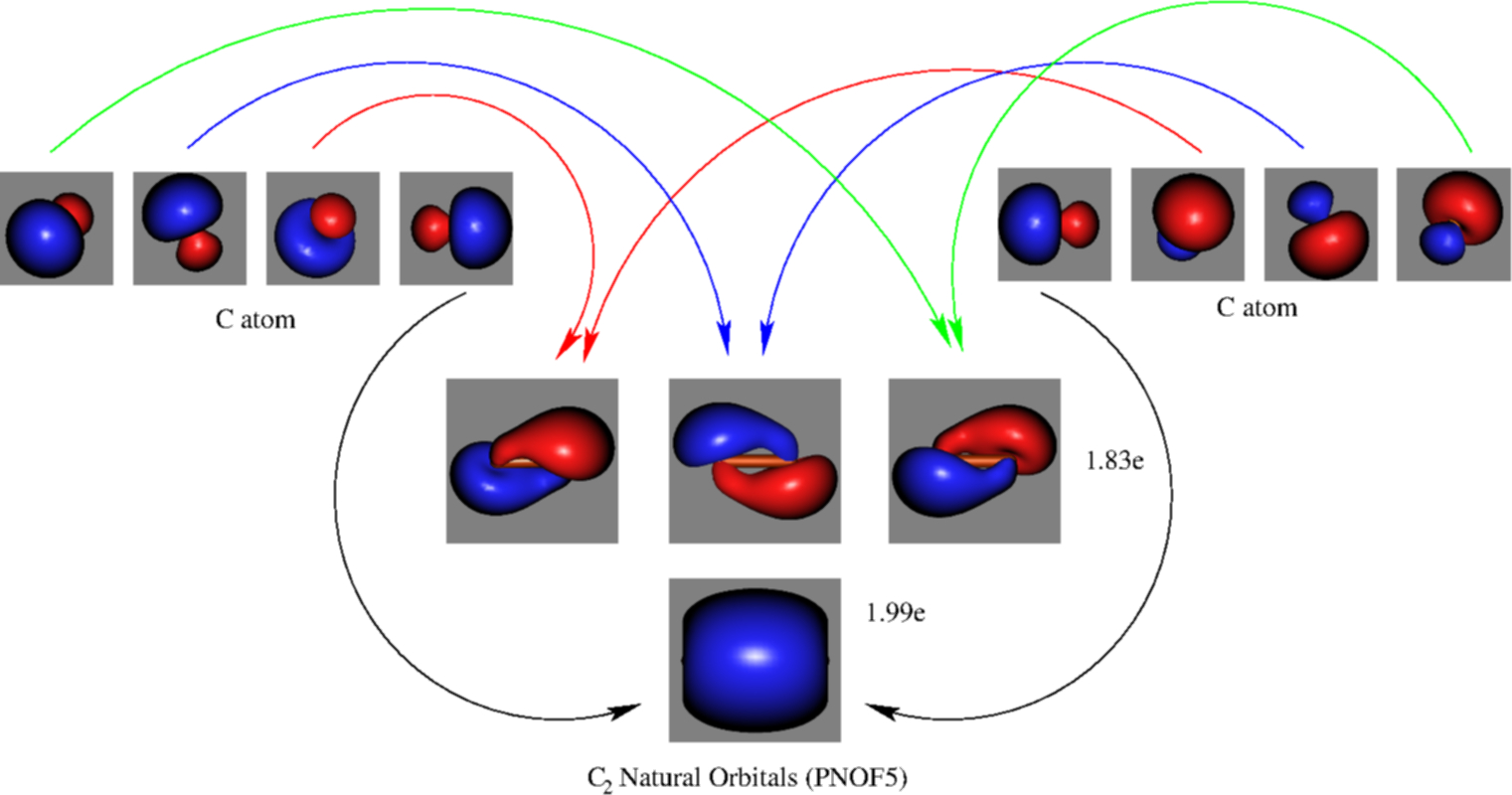
For information about the more traditional molecular structure. Molecular orbital diagram for carbon dimer (c2). Molecular orbital diagram of c 2 molecule : They combine to form the.
Li2, be2, b2, c2, n2, o2, f2, and ne2. Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using figure 9. You have the, here on this side you would have the energy, so the energy is going up there. Molecular orbital theory describes molecules in a similar way to atoms, using orbitals, orbital diagrams and electron configurations.
Valence bond (vb) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. The first major step is understanding the difference between two major theories if you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it. Bonding order is 2, and it is diamagnetic. Sigma molecular orbitals of ammonia.
• the following slide illustrates the relative energies of the molecular orbitals compared to the original atomic orbitals. And (b) he 2 in which two of the electrons figure 4. | online chemistry tutorial iit, cbse chemistry, icse chemistry, engineering and medical chemistry entrance exams molecular orbital diagram of c2 molecule : It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Mo diagram for n2+ (molecular orbital). The lowest energy unoccupied molecular orbital is 2p_(sigma), so that is where the extra electron will be added. Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.
So again, it's drawn in the familiar pattern. The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning of the twentieth century by f. Molecular orbital diagram for carbon dimer (c2). An h atom on one side and an h+ ion on the other side.
For example, to give you a glimpse at where we are headed. The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those. An mo diagram, just like an atomic orbital diagram, shows the relative energy and number of electrons in each mo. Mulliken to describe the structure and properties of different molecules.
Write molecular orbital configuration of c2+ predict magnetic behaviour and calculate its bond order. Part a by drawing molecular orbital diagrams for b2, c2, n2, o2, and item 2: Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Let me explain the molecular orbital diagram of n2 using its diagram.
• because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable.