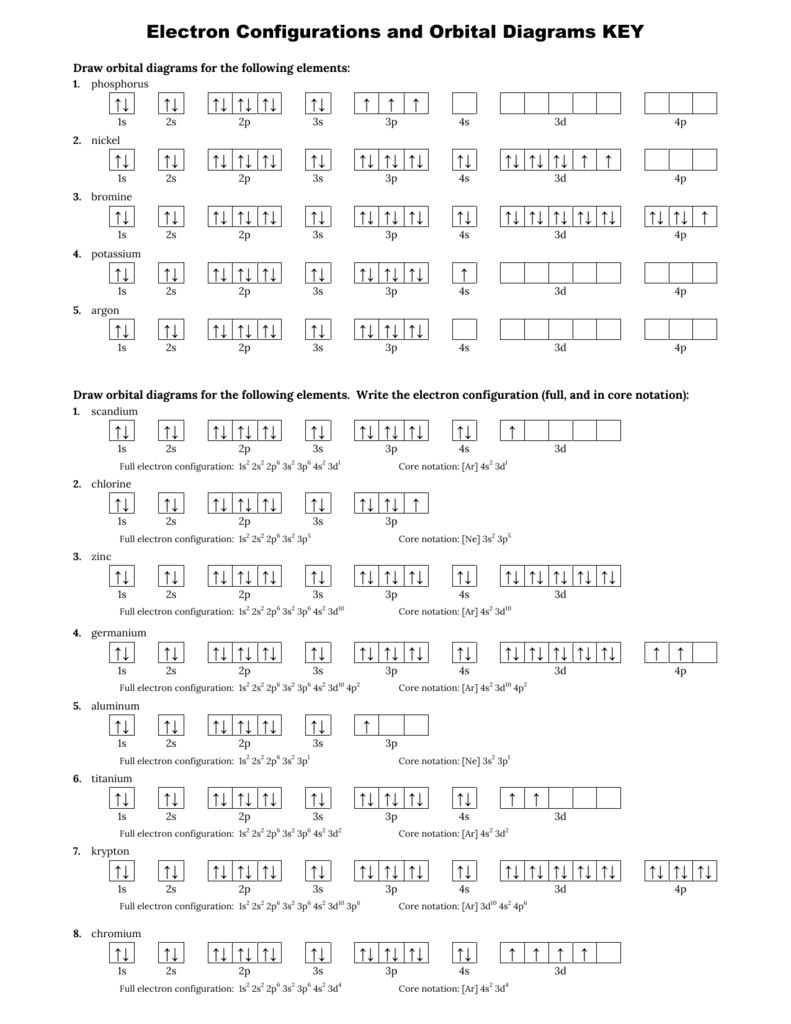
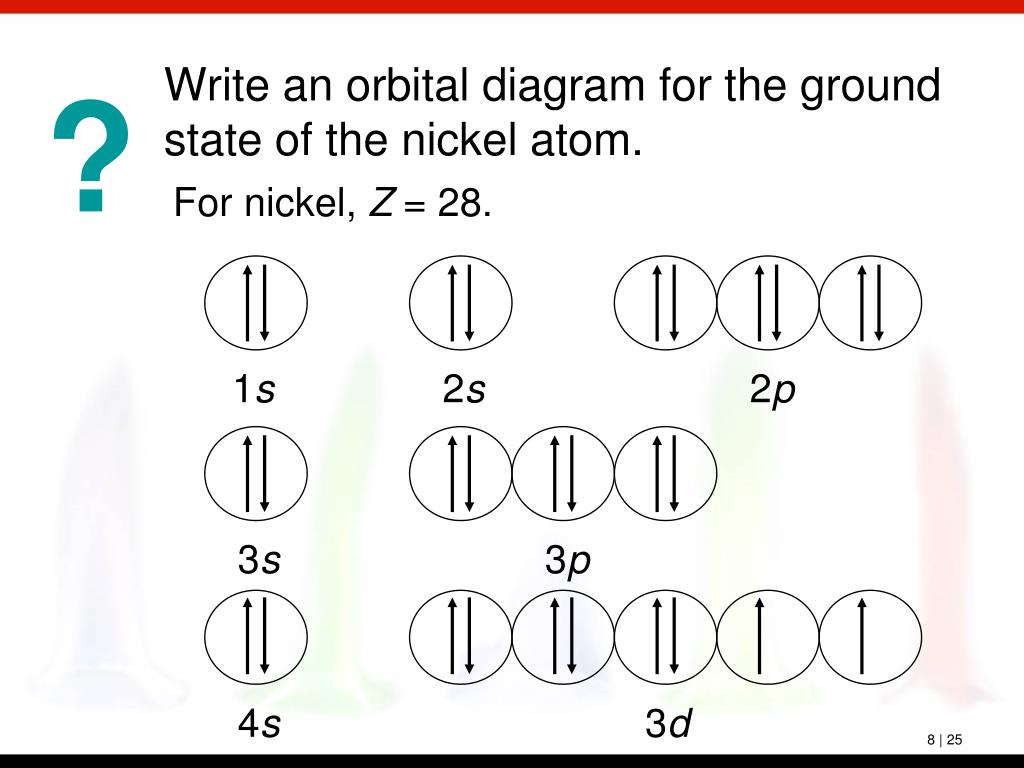
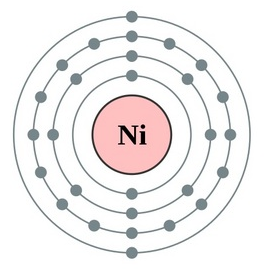
Atomic Orbital Diagram For Nickel
David's whizzy periodic table is a visual way of looking at the changing electron configuration neodymium, nd, which is used in very powerful magnets, has an atomic number of 60.
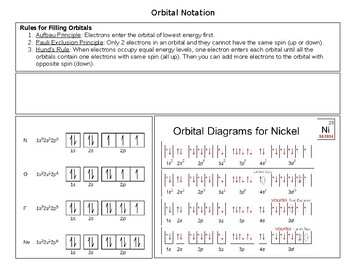
Atomic orbital diagram for nickel. Draw the atomic orbital diagrams for the two exceptions and indicate how many unpaired electrons are present. If value of bond order is. Atomic orbitals are characterized by a principal quantum number n (there are n−1 radial nodes in an ao) and a letter indicating their shape as dictated by the angular nodes: An electron in atomic orbital is under the influence of only one positive nucleus of the atom.
Figure 6.27 orbital energy level diagram for the hydrogen atom shows that the energy levels become closer and closer together as the value of n increases the energies of the different orbitals for a typical multielectron atom are shown in figure 6.29 orbital energy level diagram for a typical. So we ideally had the idea that atomic, an atom looks like and the nucleus in the middle positively charged nucleus with the electrons orbiting around it. S orbitals have a spherical shell shape and the faint dark blue circle represents in to work out an electron arrangement for an atom, you start with the atomic number, then 'fill in' the levels how do you work out the electron arrangement configuration for 28 nickel, ni ? The last electrons to be added to an orbital diagram for the atoms of the transition metal.
Encyclopedia of physical science and technology (third. The filling rules are as follows: Review of atomic orbitals for organic chemistry, s and p orbitals, electron configurations for the first 11 elements, the best graph ever, and more! This page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and its equivalents.
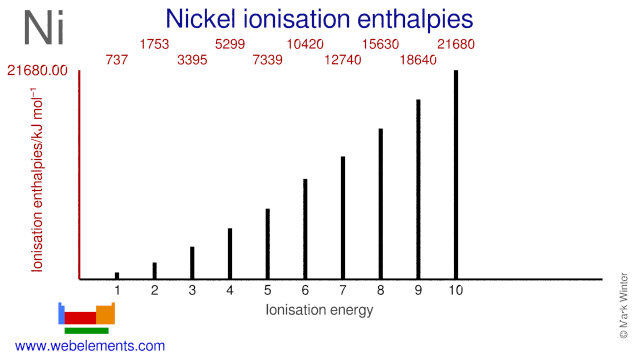
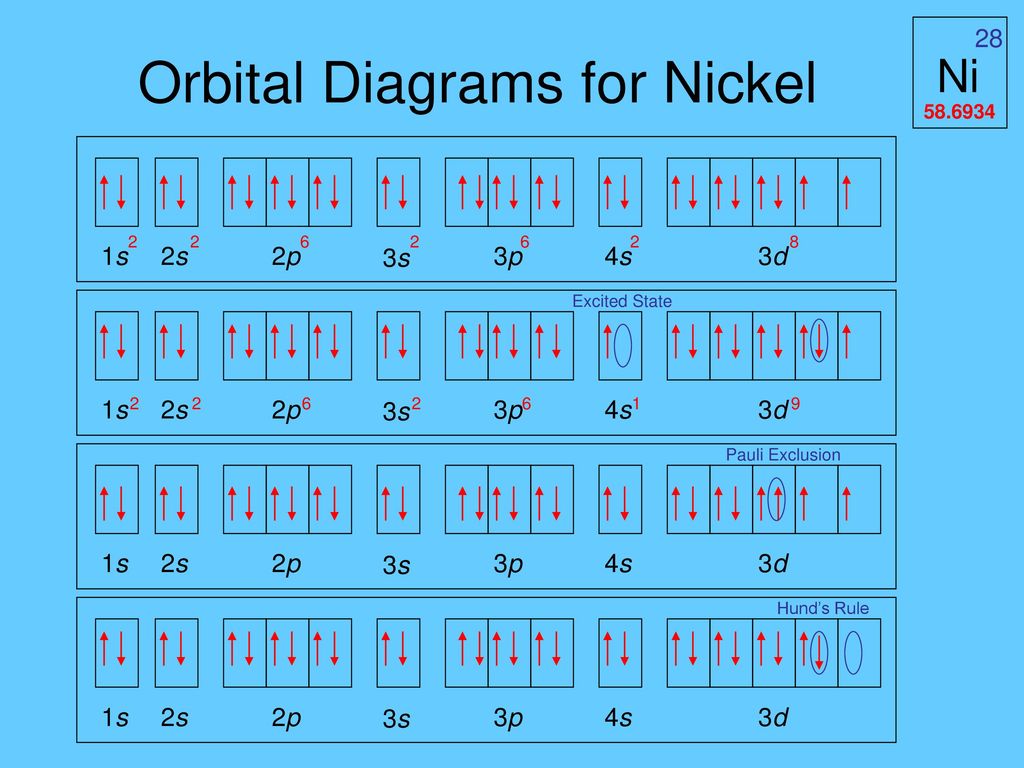
Given several orbitals at the same energy level, electrons will enter each orbital first, then add a second electron to an orbital (singles. Nickel is atomic number 28; The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (figure 8). Få 29.000 endnu en molecular orbital theory, atoms and stockvideo på 29.97 fps.
(a) complete the diagram for the 7 electrons in a nitrogen atom by. We just thought you'd like to see the elctron configuration if that element followed the rule. Download scientific diagram | molecular orbital diagrams for hbr and hf. 9 molecular orbital diagram for co.
Video i 4k og hd klar til næsten enhver nle nu. Molecular orbital energy diagrams 20. Partial orbital diagrams and condensed configurations. Therefore, it has 28 electrons in its orbitals.
An atomic orbital is defined as the region within an atom that encloses where the electron is likely the following diagram of the bohr model shows the electron existing in a finite number of allowed a diagram of the bohr model of the hydrogen atom. Like an atomic orbital, a molecular orbital is full when it contains two electrons with opposite spin. The be atom had two valence electrons, so each. An ns orbital has no angular nodes and is of spherical symmetry;
Electrons in successive atoms on the periodic table tend as the principal quantum number, n, increases, the size of the orbital increases and the electrons spend more time farther from the nucleus. Atomic orbitals are inherent property of an atom. Vælg mellem et stort udvalg af lignende scener. Refer to the related link to see an illustration of an orbital diagram for aluminum.
Valence shell orbital radii for nickel. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Electrons move in circular orbits that are at fixed. 1 atomic orbital in 3s sublevel x 2 electrons/orbital = 2 electrons can reside in the 3s sublevel.
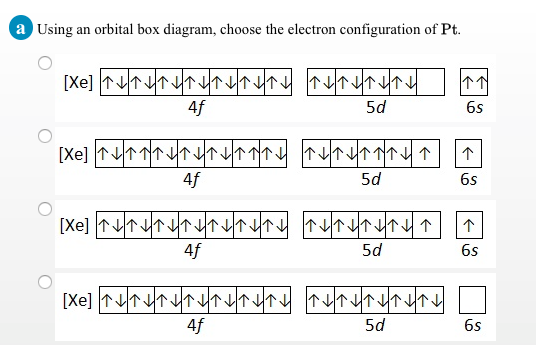
For a diatomic molecule, the atomic orbitals of one atom are shown on the left. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in each orbital. The trends in electron affinity are not as regular as those for atomic size or ie. Video explanation on atomic orbitals and the electronic structure of an atom.
This can be seen on the orbital filling diagram, but not on the electron configuration which provides less information. A partial orbital diagram shows only the highest energy sublevels being filled. The 2p, 2py, 2p atomic orbitals are degenerate in an atom and have been separated for. Each of the two sp hybrid orbitals holds one electron when atomic orbitals hybridize, the valence electrons occupy the newly created orbitals.
Atoms with a high ea tend to form anions. Mann, atomic structure calculations ii. An atomic orbital may contain 2 electrons at most, and the electrons must have different. Thus the two electrons occupying the 1s orbital must have different spins.
Hybridization of atomic orbitals, sigma and pi bonds, sp sp2 sp3, organic chemistry, bonding. When you input the atomic number of one of these elements, you will get the incorrect configuration displayed in the first row and the correct configuration will be shown in row 3. Lowest energy levels fill first. Analysis done by bond order.
Here's a diagram of the first several electron configurations. The following article is intended as a quick review on atomic orbitals for students enrolled in an introductory organic chemistry class who are assumed to.