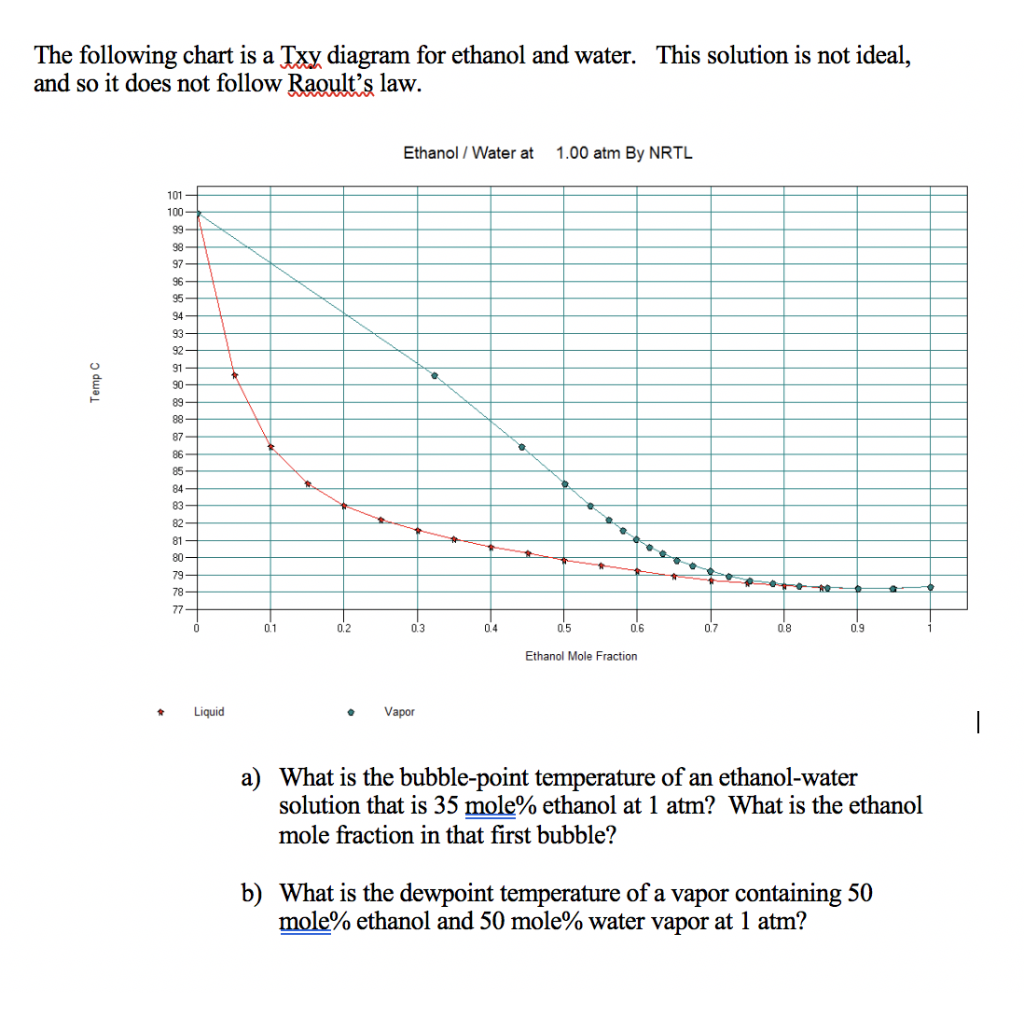
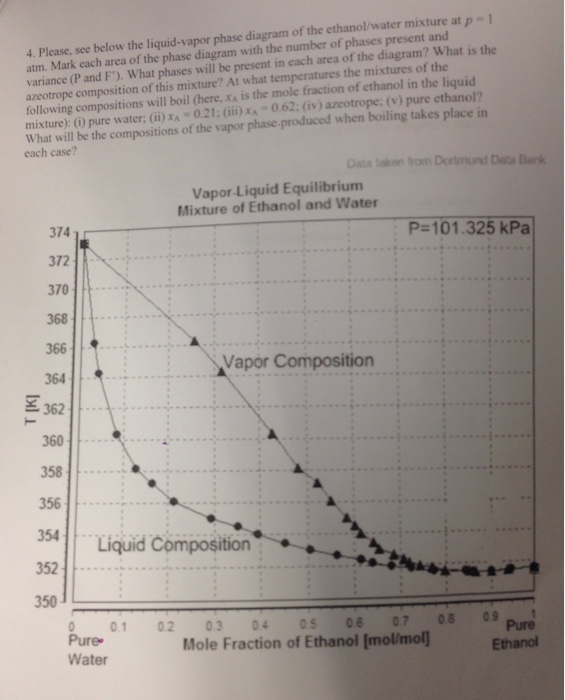
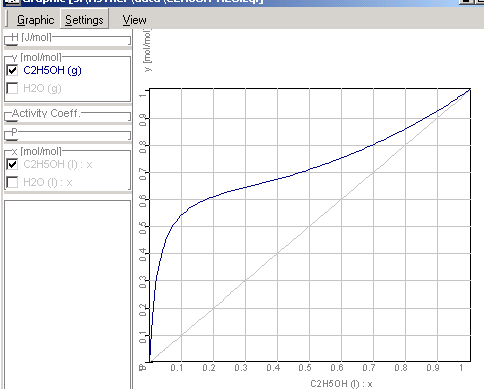
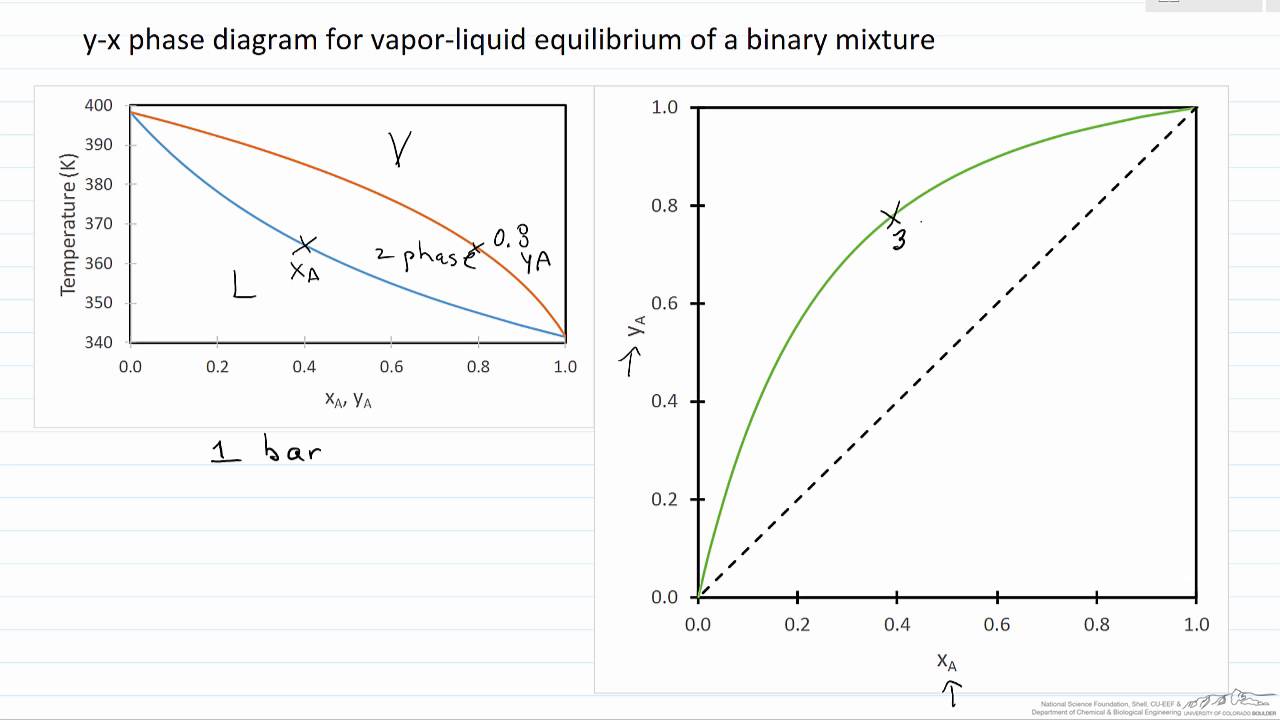
Xy Diagram Ethanol Water
If anyone has any information on where i can find one or if anyone has one , it will be greatly.
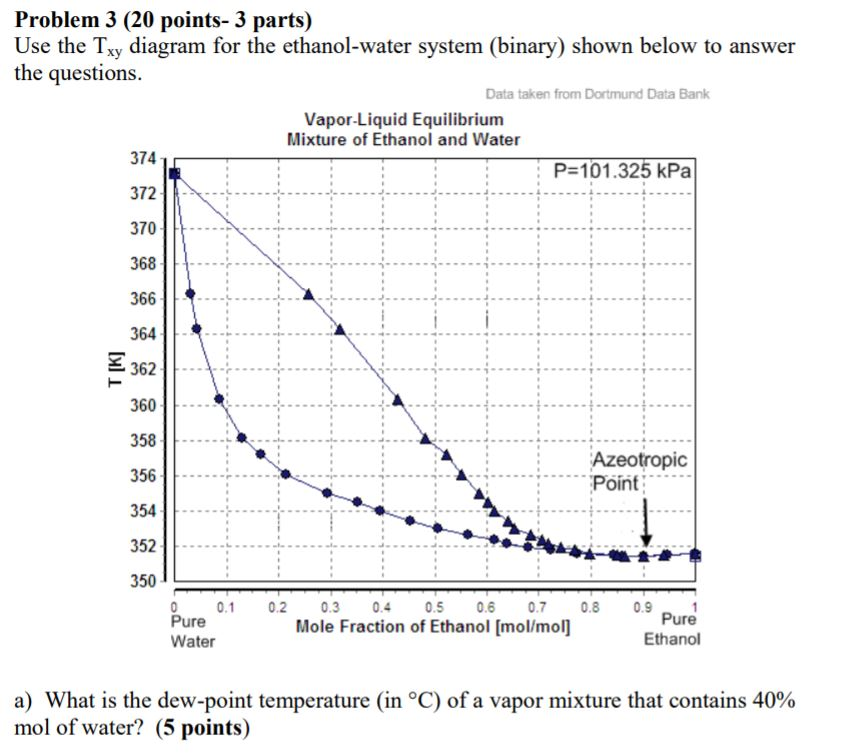
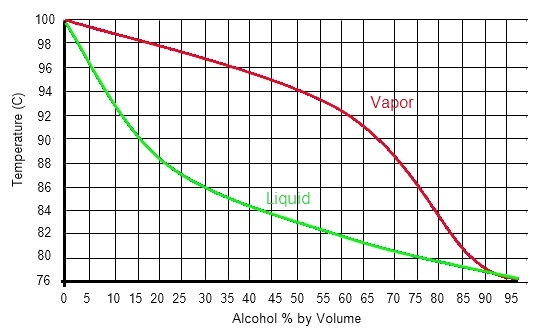
Xy diagram ethanol water. These data correlate as ρ [g/cm3] = −8.461834×10−4 t [°c] + 0.8063372 with an r2 = 0.99999. Shown on the diagram are the vle, operating and ideal lines. The purpose would be to be able to boil the ethanol off at a lower temperature to preserve the quality of the contaminant. You need to make sure.
0,65 vapor mole fraction, ethanol. Explains how to generate phase diagrams and read them for use in separation calculations. Reorientation motions of water and ethanol become slower as ethanol concentration is increasing. Made by faculty at the university of colorado boulder, department.
You may receive emails, depending on your notification preferences. Bilel hadrich and nabil kechaou. There is a lot of information on this diagram, so we will first identify some of the lines on the diagram. Xy data for ethanol / water wilson parameters:` 276.760` 975.490 t deg f p psia x1 y1 gamma1 gamma2.
Ratings 100% (1) 1 out of 1 people found this document helpful. ( i selected ethanol and water as my two components, and remember that there can be multiple components in your system, and when making your xy step12: This page provides supplementary chemical data on ethanol. The othmer paper is attached for additional.
Except where noted otherwise, data relate to standard ambient temperature and pressure. The student should then test themselves on their understanding of the diagram by trying the interactive questions below to the right of the diagram. Water are assumed to be present in the vapor distillate stream coming out double effect column. Were in the process of getting our solvent recycler to work with the vacuum distillation of ethanol.
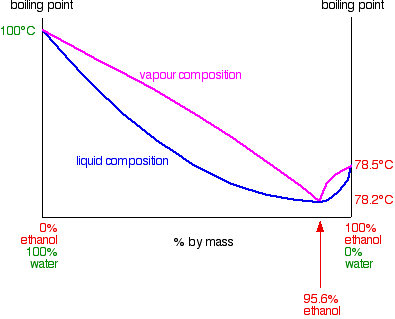
Ethanol produced by the fermentation process is so far the. Electron configuration of the elements. The density is given as g/cm3. The ethanol + water diagram is shown below.
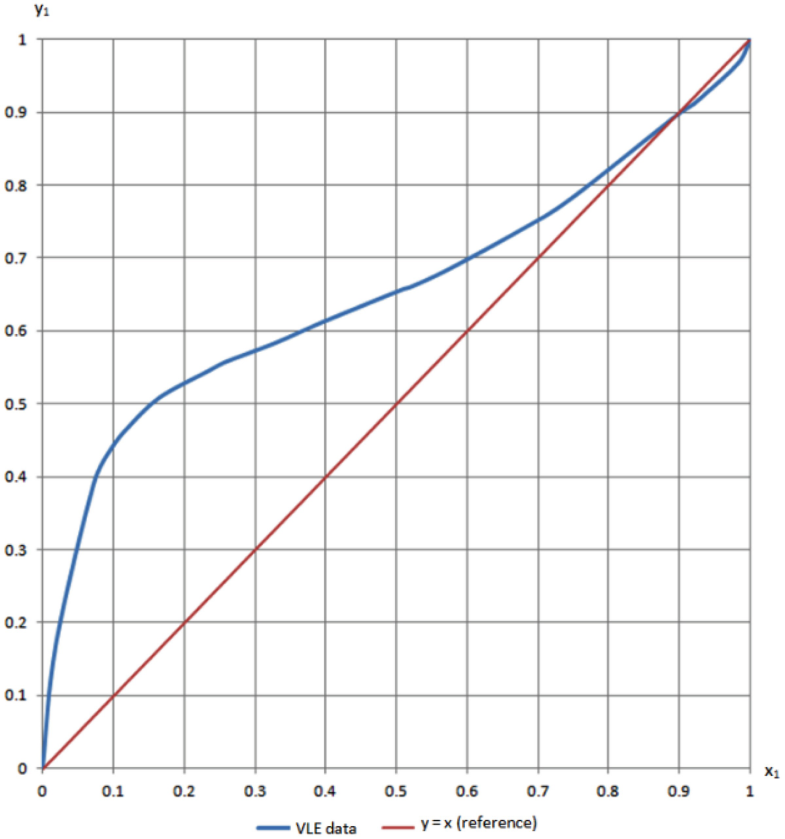
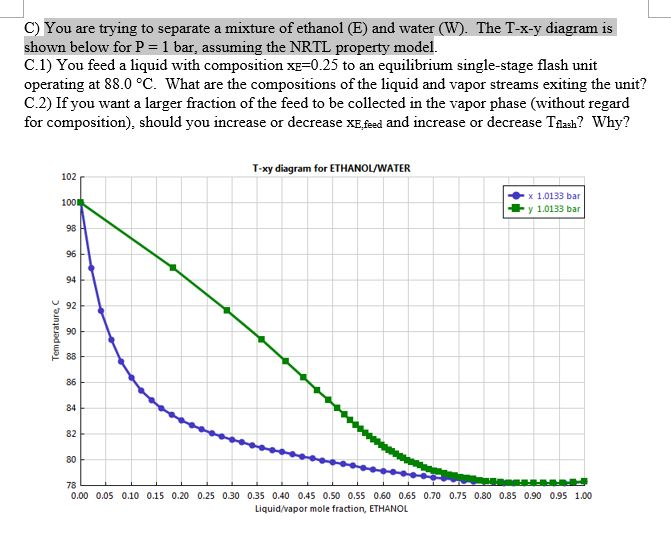
I've not been able to get one. You will get a typical txy diagram, depending on your components and property method, but this is not the same as the xy diagram we. Figure 1.14 txy diagram for ethanol/water (a), activity coefficient plot (b), and xy plot (c) for ethanol/water. % 3 84 liquid mole fraction (etoh) your objective is to achieve the best separation transcribed image text from this question.
So i can see that enthalpy of water less then enthalpy of ammonia (at the same pressure, vapor fraction =0). Shown on the diagram are the. } add approximately 30 ml of deionized water to boiling chamber a. there is no need to add cold tap water to condenser d at this time. Density and composition of some ethanol water mixtures.
We also draw the tie lines and the conjugate line. Enthalpy is expressed in kcal/kg and composition is expressed in weight fraction of ethanol. Why i get this result? Each point corresponds to a different temperature.
I have found some diagrams in the internet.