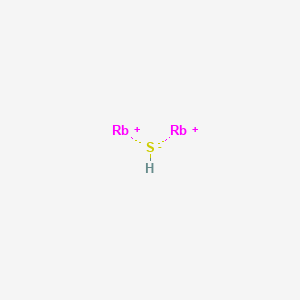Rubidium Lewis Dot Structure
This page is an index list of all of my lewis dot structure videos.
Rubidium lewis dot structure. So nitrogen (n) would look like this There are many exceptions to this rule, but it should be used as a general guide for creating lewis structures. A lewis structure is a structural representation of a molecule where dots are used to show electron position around the atoms. Or maybe you use a chemistry sketch program and wish there was an easier way?
This clipart collection contains images. In chemistry, drawing lewis dot structures can be challenging, but they provide a wealth of information about the molecules they represent. How to draw lewis structures : How do i draw the lewis electron dot structure of cyanamide cn2h2?
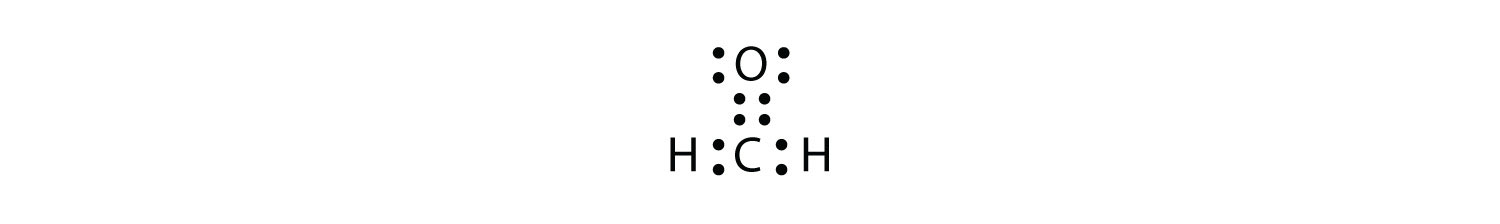
Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping. Electron dot (lewis dot) structure for rubidium. If you are required to teach lewis dot structures, this short lesson can help you extend what students have learned about modeling covalent and ionic bonding.
Lewis structures have a central atom and a terminal atom. Lewis dot structure is the classical bonding model in which only valence electrons of the atoms are used. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. 1280 x 720 jpeg 63 кб.
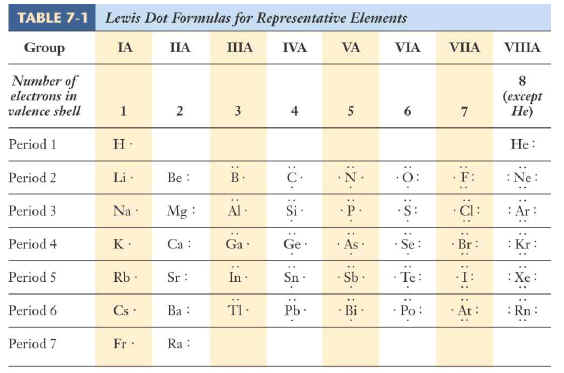
Remember that lewis dot structures are drawn for covalent (molecular) compounds that share electrons. Anyway, a good counting place on the periodic table would be the second row (or period). How to analyze different ways to draw the dot structure for sulfur dioxide. Lewis symbols of the main group elements.
We can learn to make accurate lewis dot structures in 4 simple steps. Rubidium (rb) element definition, symbol, isotopes, properties (electron configuration, atomic number and mass, valence electrons, density), what is it used for. 20 lewis dot structures valence electrons are very important in chemical compound formation so it helps if we can indicate these electrons schematically these drawings are called lewis dot structures. If you use the cross over method it would be rb:h, but then rubidium doesnt have a full octet.
Count the number of valence electrons from atoms in a molecule. The structure on the right is the lewis electron structure, or lewis structure, for h2o. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. One of the early questions asked by scientists, once the concepts of atoms and molecules had been firmly established was how are atoms bonded? we've developed many theories over the years in attempts to explain the bonding between atoms in various substances.
Lewis structure is basically a graphic representation of the electron distribution around an atom. Aren't you sick of drawing all those lewis dots on your diagrams after you print out your worksheets, tests, or quizzes? They are created by placing the valence electrons around the elements symbol. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired.
Rb is the short form of rubidium. Lewis structures incorporate an atom's formal charge, which is the charge on an atom in a molecule. The dot structure as you know can only be a max of 8 and dots are added counterclockwise. Sulfur hexafluoride sf6 lewis dot structure.
Lewis dot structures help predict molecular geometry. Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. In lewis dot structures each dot represents an electron. I show you where rubidium is on the periodic table and how to determine.
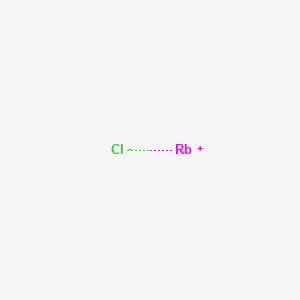
With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. It will be necessary to create a double bond between an oxygen (o) and a nitrogen (n) atom for the lewis structure to work. The major reason why learning lewis dot structure is important is that it helps in predicting the number and type of bonds which can be formed around an atom. How to draw the lewis dot structure for rbcl:
The dot structure for rubidium is rb with a dot on the top right of b. The lewis formula is used to describe covalent bonds. Lewis structures, also called lewis dot diagrams, model covalent bonding between atoms. These diagrams use dots around atoms to signify electrons and lines to signify bonds between atoms.
480 x 360 jpeg 14 кб. Rubidium electron configuration (bohr model). How to draw lewis structures. Also note that you should put the no3.
In describing lewis's structure the steps that can be taken are: Lewis structures first came into use early in the twentieth century when chemical bonding was poorly understood. Lewis dot structure of atoms link. Draw lewis dot structures for each of the following atoms predict the common oxidation numbers for each of the following elements when they form ions (there may be more than one):
20 elements, dots, and atoms! Lewis dot structures (or just lewis structures) were developed around 1920 by pioneering chemist gilbert lewis, as a way of picturing chemical bonding in molecules. These steps are easy to understand and implement. Electron dot diagrams help illustrate electronic structure of molecules and chemical reactivity.