Ne2 2 Molecular Orbital Diagram
Molecular orbitals mo are constructed from atomic orbitals.
Ne2 2 molecular orbital diagram. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. To see how this works, we will consider the simplest possible molecule, h2+. One is for the elements up to nitrogen. We can see this by a consideration of the molecular electron.
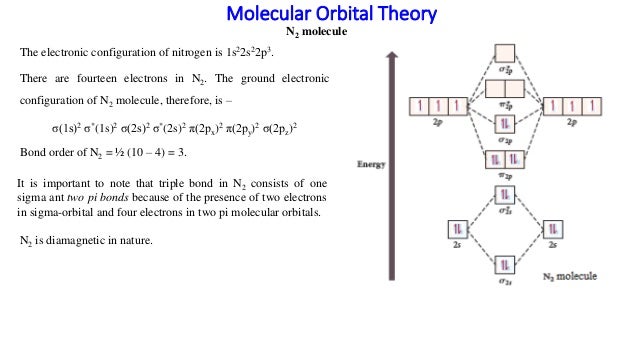
They combine to form the molecular orbitals indicated above. Molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms, that is, in terms of orbitals, orbital diagrams, and when two larger atoms atoms combine to form a diatomic molecule (like o2, f2, or ne2), more atomic orbitals interact. Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion. The bond order of is, 3.
The video below describes how to generate molecular orbital diagrams for b₂ and other diatomic molecules from row 2 elements of the. Mo diagram for n2+ (molecular orbital). Let me explain the molecular orbital diagram of n2 using its diagram. | online chemistry tutorial iit, cbse chemistry, icse chemistry, engineering and medical chemistry entrance exams molecular orbital diagram of c2 molecule :
There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). A molecule in which all the electrons are paired, is called diamagnetic. If 2p orbitals on an atom are all the same energy, why do they form molecular orbitals of different engergies when theu mix? Write the ground state molecular orbital electron contiguration | molecule enetsy level diagram including each atom's energy levels) molecular orbital clectron.
What is the atomic and molecular orbital diagram of f2. Mo diagram for n2+ (molecular orbital). N2+ has a weaker longer bond than n2, but o2+ has a stronger, shorter bond than o2. Eight electrons from each oxygen atom add up to 16 electrons in the o 2 molecule.
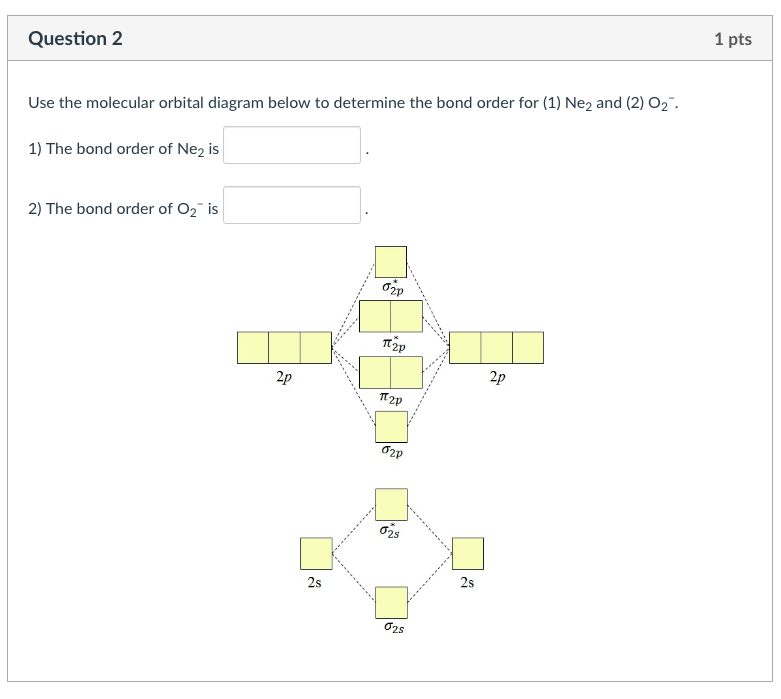
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Neon atom has 10 electrons and its electronic configuration is. Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the pauli principle, the what is the molecular orbital diagram for the diatomic neon molecule, ne2? Mo energy diagram for o 2.
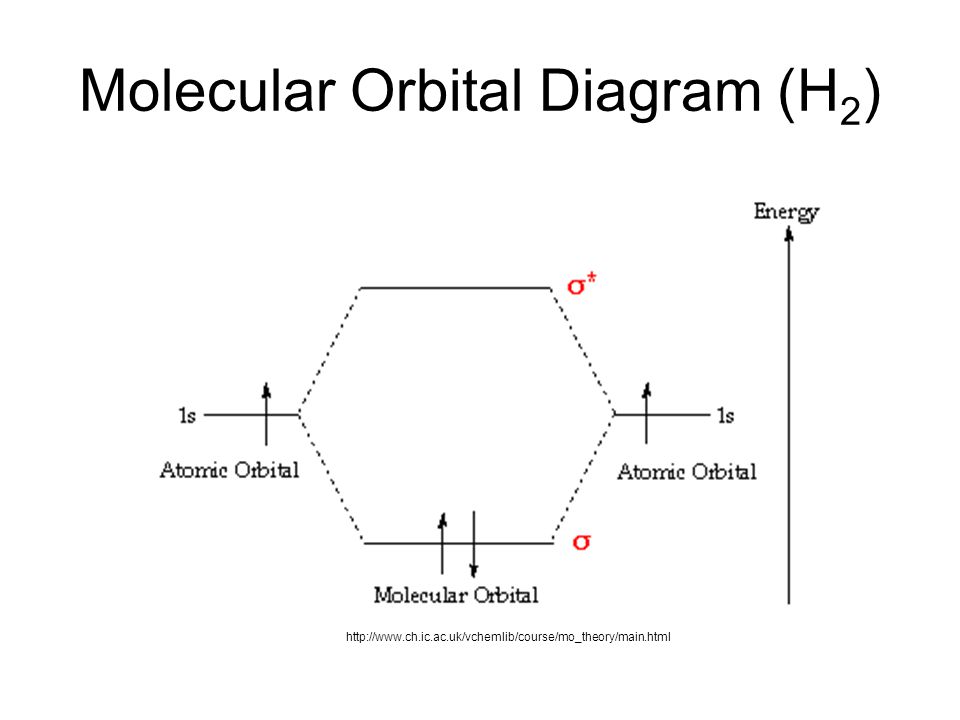
Molecular orbital energy diagrams for (a) h 2 showing both electrons in the bonding sigma mo; As the bond order value for molecule is zero, it is unstable and cannot exist. Now note that even in this advanced molecular orbital theory a bunch of approximations is introduced, and the answer in general depends on at which level of theory calculations are done. Construct a molecular orbital diagram for the hydrogen molecule ion h2+ by using.
Each molecular orbital can only have 2 electrons, each with an opposite spin. And (b) he 2 figure 4. However, we can predict that the be2 molecule and the ne2 molecule would not be stable. Number of electrons in c2 molecule = 12.
Dihydrogen and its ion h2+. This is the molecular orbital diagram for the homonuclear diatomic be2+, showing the molecular orbitals of the valence shell only. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2). The molecular orbital diagram of hypothetical molecule is given in the attachment.
Interactive video lesson plan for: The first major step is understanding the difference between two major theories if you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it. 24:30 tricky chemistry basics by suman negi 150 442 просмотра. How to write simple molecular orbital diagrams and determine the bond order.
This is the hydrogen molecule ion, which consists of two nuclei of charge +1, and a single electron shared between them. The other is for after nitrogen. According to the molecular orbital theory, the general molecular orbital configuration will be, as there are 7 electrons present in nitrogen. Draw the molecular orbital energy level diagram for the following substances, and complete the tables.
There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). B, c, n, o, f, ne. Molecular orbital mo diagram of n2 molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you sigma2s 2 sigma2s 2 pi2p 4 mo diagram for n2 molecular orbital there are two mo. Molecular orbital diagrams, bond order, and number of unpaired electrons.
Draw the molecular orbital diagram for the oxygen from the molecular orbital diagram of n2, predict its bond order and whether it is diamagnetic or paramagnetic. An mo diagram, just like an atomic orbital diagram, shows the mo occupancy and molecular properties for b2 through ne2. One atom of nitrogen has 7 electrons so a n2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bon. If 2p orbitals on an atom are all the same energy, why do they form molecular orbitals of different engergies when theu mix?
Will the mo diagram be the same as that of $\ce{n2}$ or not?