Mo Diagram Of Ne2
Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2).
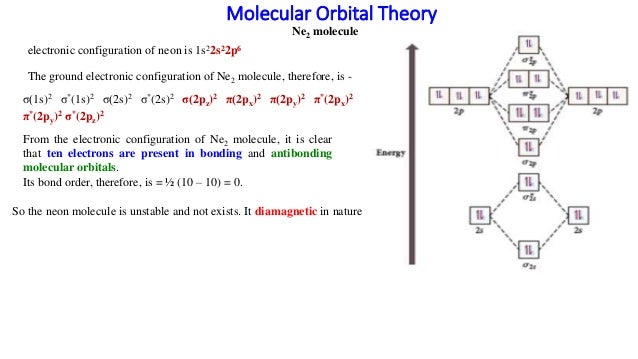
Mo diagram of ne2. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Bonding order is 2.5, so it is. Li2, be2, b2, c2, n2 o2, f2, ne2. We will predict their bond order and see how the energies of the different orbitals change.
3.1.5 significance of the sign of the wavefunction. We will also compare our predictions to experimental evidence. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2). Calculate the mean free path of n2 molecules at 350k under … these conditions.
From the molecular orbital diagram of n2, predict its bond order and whether. F2 mo diagram 2 free wiring diagram for you. The mo diagram of hcl that includes all the valence orbitals of the cl atom is shown in fig. In dineon ne2 (as with dihelium) the number of bonding.
- Ttr 125 Carburetor Diagram
- 2004 Explorer Radio Wiring Diagram
- Badland Winch Wireless Remote Wiring Diagram
Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wavefunctions. However, we can predict that the be2 molecule and the ne2 molecule would not be stable. Molecules of the first row Mo diagrams look like this:
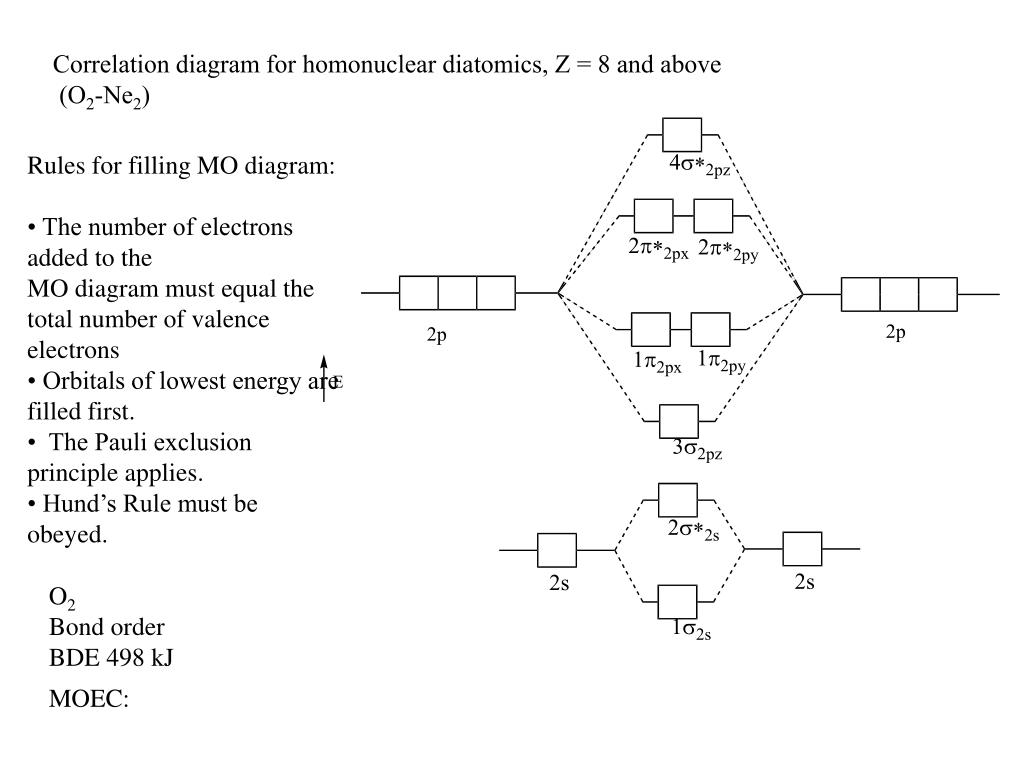
Cnx o2 molecular orbital diagram wiring diagram and electrical. Neon atom has 10 electrons and its electronic configuration is. When it comes to o₂ and n₂, i think there are two things to point out Electrons dance to a subtle.
Probability density curve for h2 molecule. B2 c2 n2 o2 ne2 f2 4. The molecular orbital diagram of are shown below. As shown in table 3, be2 and ne2 molecules would have a bond order of 0, and these molecules do not exist.
You've seen the molecular orbital (mo) diagram of #co_2#: Mo for n 2 chemistry stack exchange. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2). The other is for after nitrogen.
Molecular orbital diagram for nitrogen gas (+1 ion) (n2(+)). One is for the elements up to nitrogen. Fill from the bottom up, with 9 valence electrons total. Mo diagram a molecular orbital diagram or mo diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular.
Transcribed image text from this question. Mo diagram f2 (5) complete the following table (calculate all the quantities by considering by neglecting the 1s shell). 27.02.2013 · there are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). Molecular orbital mo diagram of n2.
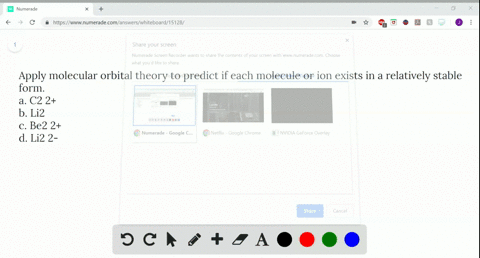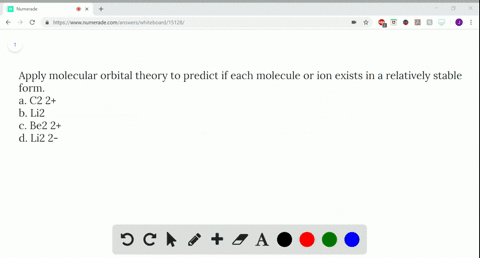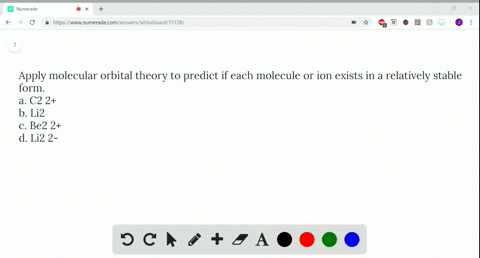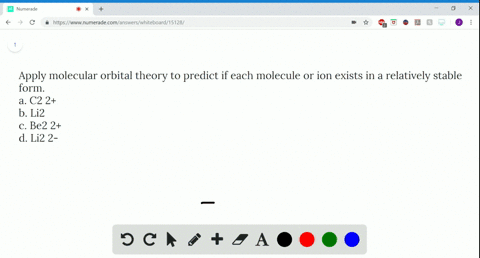
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. The following molecules are currently available: One is for the elements up to nitrogen. 2 + draw the molecular orbital (mo) electron diagram for the ne 2 molecular ion.
Posted on september 15, 2014 by admin. Mo diagram of o2 and n2. Bond order is 3, and it is paramagnetic. Note the mo diagram is different here than it is for o2/f2/ne2.
For now, we're only covering homonuclear mo diagrams which involve the diatomic molecules composed let's calculate the bond order of `ne_2`. The bond order of is, 3. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. #co_2# and #no_2^+# are isoelectronic and thus have the same electron configuration.
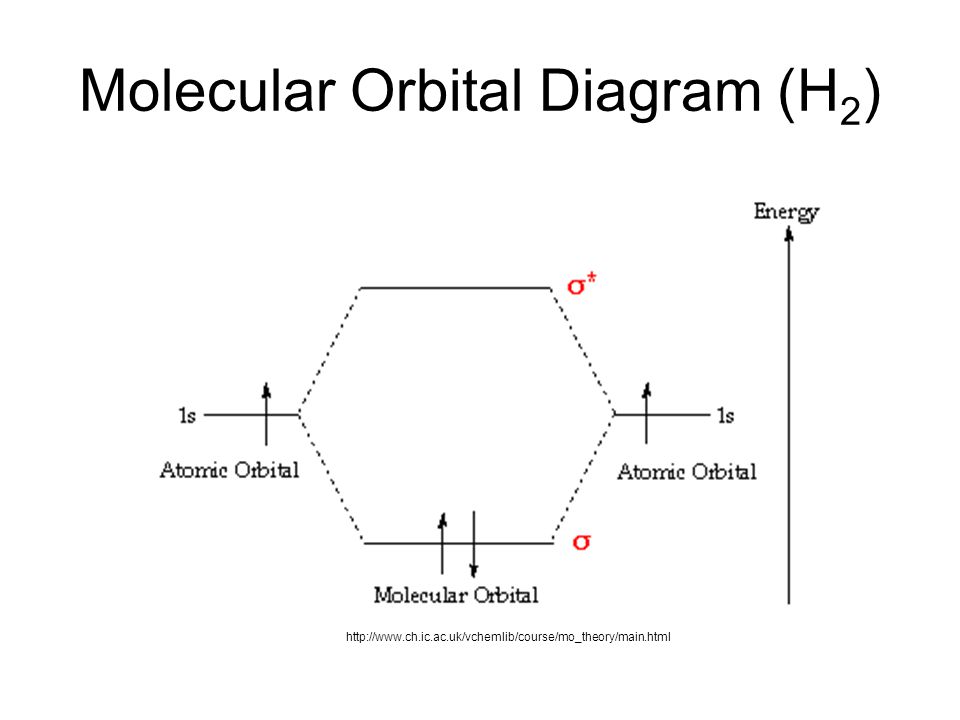
If we compare such diagrams for the diatomic molecules on the second period (li₂, be₂, b₂, c₂, n₂, o₂, and f₂), the resulting pattern looks like this: There are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc. Simple mo diagrams for the overlap of two 1s aos, e.g., for h2+. They're not as intimidating as they may seem.
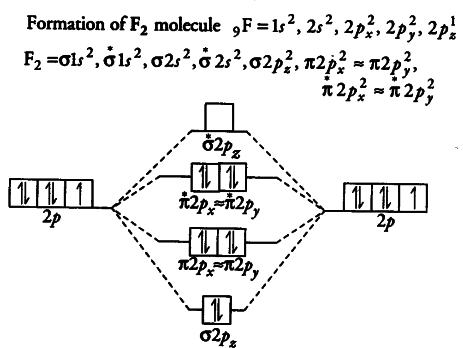
Ne2 molecular orbital diagram periodic table q inspirationa. Using the mo diagrams shown in figure 11, we can add in the electrons and determine the molecular electron configuration and bond order for each of the diatomic molecules. There are `8` electrons in bonding orbitals and `8` electrons in antibonding orbitals. In difluorine two additional electrons occupy the 2pπ* with a bond order of 1.
Which has the shortest bond? Whilst this is the mo diagram for n₂: Two of the cl valence orbitals (3px and 3py) have the wrong symmetry to interact with the h 1s orbital. There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc).