Mo Diagram For Ne2
Determine point group of molecule (if linear, use d2h and c2v instead of d∞h or c∞v).
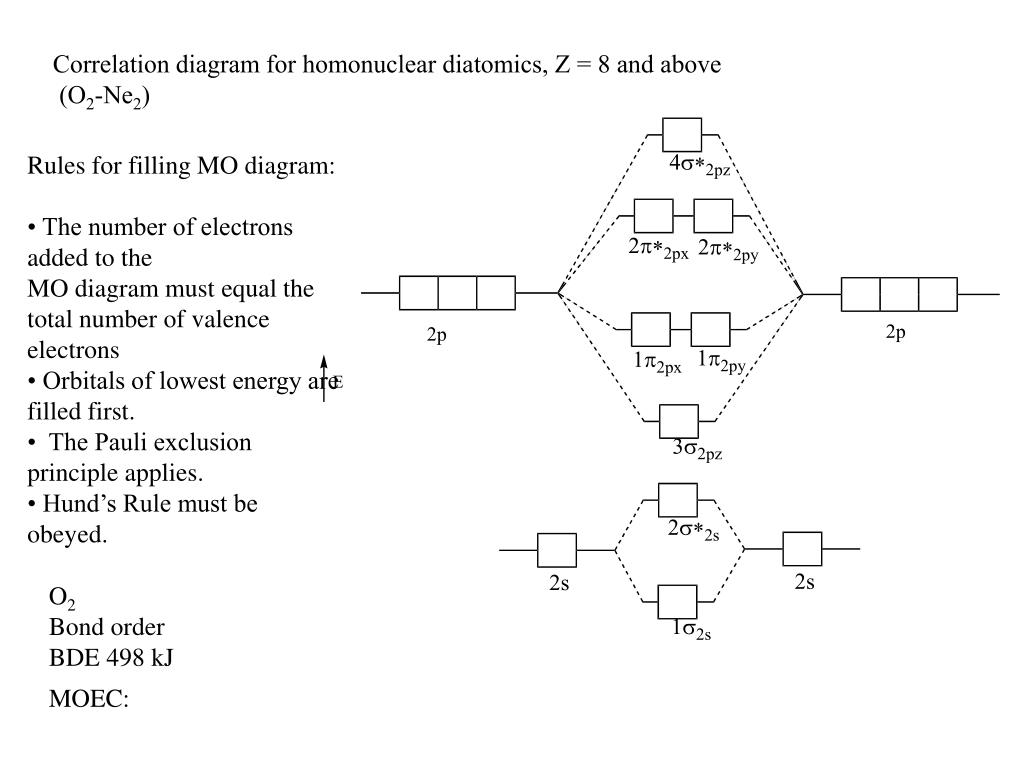
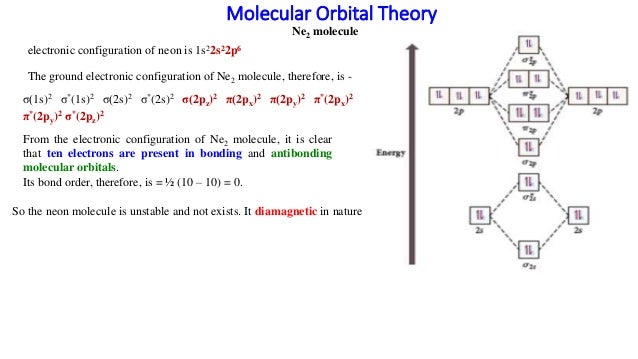
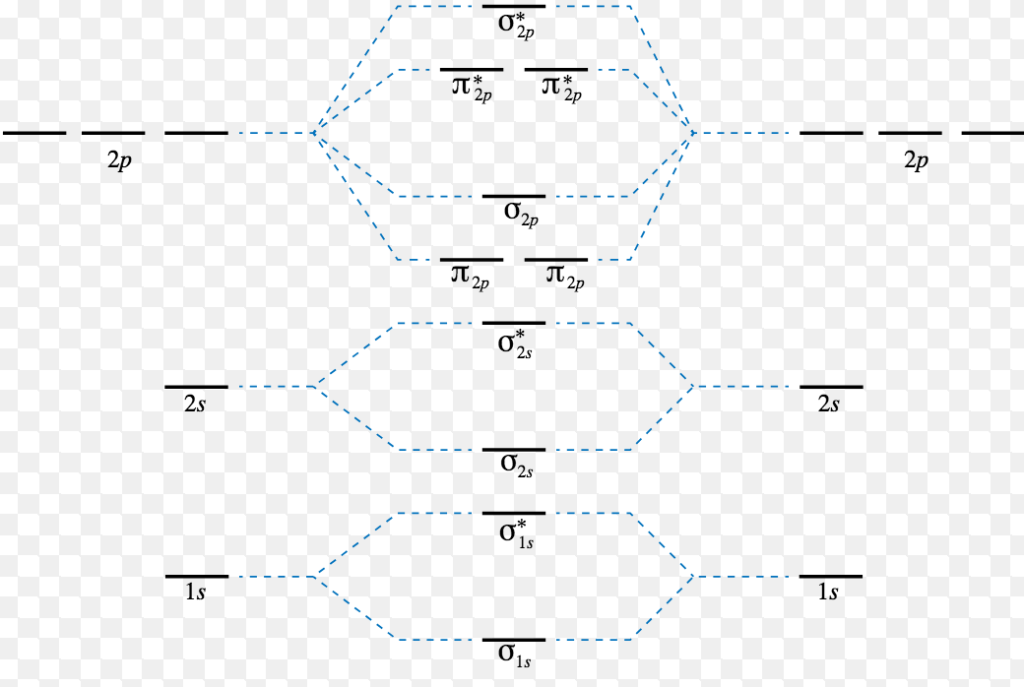
Mo diagram for ne2. #co_2# and #no_2^+# are isoelectronic and thus have the same electron configuration. We will also compare our predictions to experimental evidence. There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). The mo energy level diagram does not show the 1s orbitals.
Mo diagram o2 o2 2 o2 o2 2 preparation of gate csir net uset set exam. Assign x, y, z coordinates (z axis is principal axis; The diagram is then completed by filling the energy levels with the correct number of electrons. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram.
Transcribed image text from this question. Σ1 mo diagram of co2 molecule. Mo diagram for n2+ (molecular orbital). Mo diagrams look like this:
- 1989 Ford F150 Fuse Box Diagram
- 1996 Jeep Grand Cherokee Fuse Box Under Hood
- 2010 Dodge Caliber Sxt Fuse Box Diagram
We will predict their bond order and see how the energies of the different orbitals change. Transformation matrix from atomic basis to molecular/qa orbitals. Molecular orbital problems 1.a molecular orbital (mo) energy level diagram appropriate for homonuclear diatomic molecules from li2 to n2 is shown below. This shows two unpaired electrons in π2px and π2pz.
Contribute to kumrud/mo_diagram development by creating an account on github. 3.1.5 significance of the sign of the wavefunction. They're not as intimidating as they may seem. The other is for after nitrogen.
When it comes by using molecular orbital theory, why doesn't the ne2 molecule exist? Whilst this is the mo diagram for n₂: If we compare such diagrams for the diatomic molecules on the second period (li₂, be₂, b₂, c₂, n₂, o₂, and f₂), the resulting pattern looks like this: Interactive video lesson plan for:
Diagram for b2, this is what you get: Molecular orbitals for larger molecules. Class 11 chap 4 chemical bonding 11 molecular orbital theory iit jee neet mot part ii. Mo diagram for triangular h.
Be sure your diagram contains all of the electrons in the molecule, including any core electrons. There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). Explain why the 2p orbitals are shown to be involved in the. One is for the elements up to nitrogen.
Draw the molecular orbital (mo) electron diagram for the li2 molecule. You've seen the molecular orbital (mo) diagram of #co_2#: For $\ce{n2}$ the orbitals in increasing energy are To further demonstrate the consistency of the lewis structures with mo.
For now, we're only covering homonuclear mo diagrams which involve the diatomic molecules composed let's calculate the bond order of `ne_2`. Draw mo diagram for be 2 : Mo diagrams for linear and bent molecules. In dineon ne2 (as with dihelium) the number of bonding.
I have been taught that the mo diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. Draw the mo diagram for ne2 and determine the bond order. There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). One is for the elements up to nitrogen.
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Mo diagram for hf the ao energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. Molecules of the first row The following molecules are currently available:
The energy ordering for b 2 , c 2 , and n 2 energy ordering for o 2 , f 2 , and ne 2 notice the example use molecular orbital theory to determine the bond order of ne 2 second=period heteronuclear diatomic molecules fluorine is so. There are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc. Electrons dance to a subtle. When you write the m.o.
27.02.2013 · there are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc). Chemical bonding drawing the mo energy diagram for a period 2 homodiatom draw the molecu lar orbital (mo) electron diagram. Accurate mo calculations provide the total electron density and predict observable properties (vibrations, nmr, electronic transitions, magnetism) mos have the symmetry of the irreducible representations maximizing delocalization lcao mos give us the means. One is for the elements up to nitrogen.
There are `8` electrons in bonding orbitals and `8` electrons in antibonding orbitals. Here is the mo diagram for o₂: The molecular orbital energy level diagram for both the no and cn ions. In difluorine two additional electrons occupy the 2pπ* with a bond order of 1.
As discussed in class the mo diagram for b2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding. Mo diagram a molecular orbital diagram or mo diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular. Page 11 draw mo diagram for li 2 : Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).