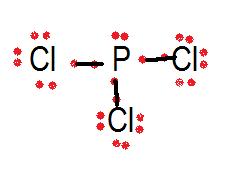
Lewis Structure Of Pcl3
We then combine electrons to form covalent bonds until we come up with a lewis structure in which all of the elements.
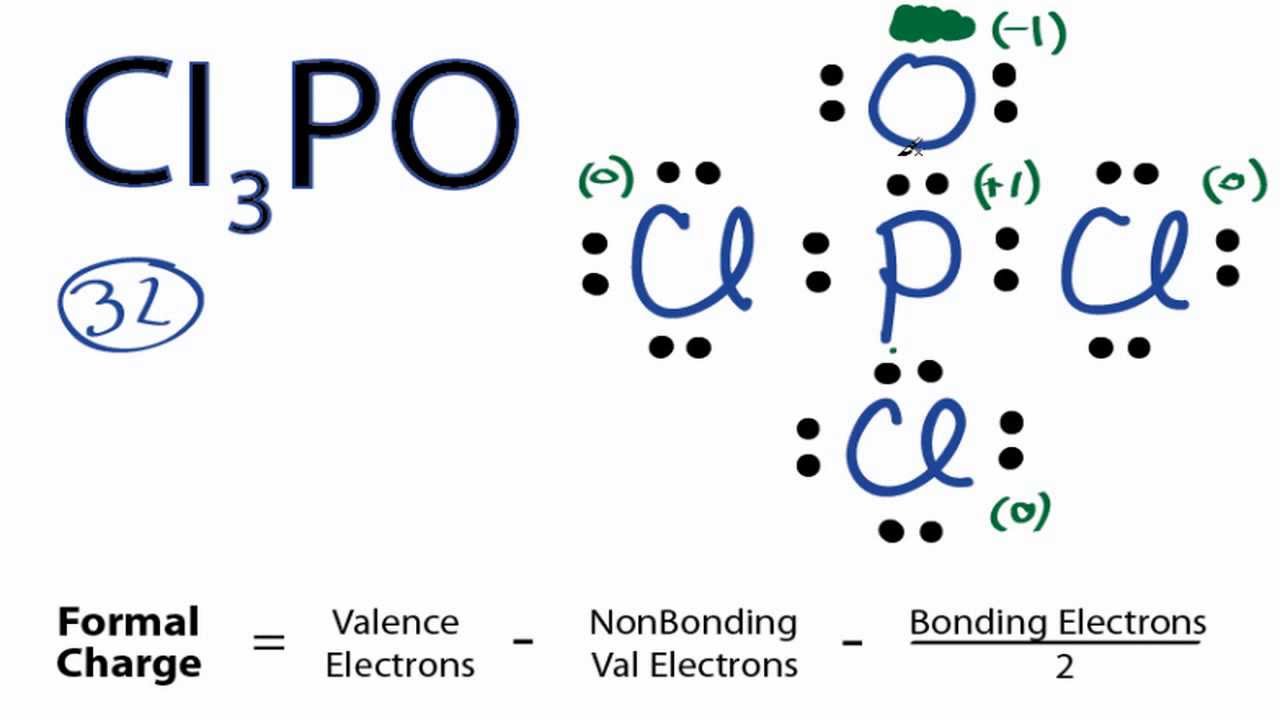
Lewis structure of pcl3. For the pcl3 lewis structure we first count the valence. These atoms can exceed 8 surroundings electrons in the structure. The lewis structure of water shows that the oxygen atom has two lone pairs. Write lewis structures for the following:
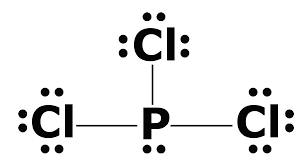
Crystallographic data and structure refinement for [pcl215n]3, [pcl215n]3.alcl3, [pcl215n]3.albr3, and. If you consider the axn method, the a should be found as the central phosphorus atom, and the x is the number of particles pcl3 bond angles and shape. Here is the lewis structure of pcl3: 3.1 lewis structures and vsepr theory?
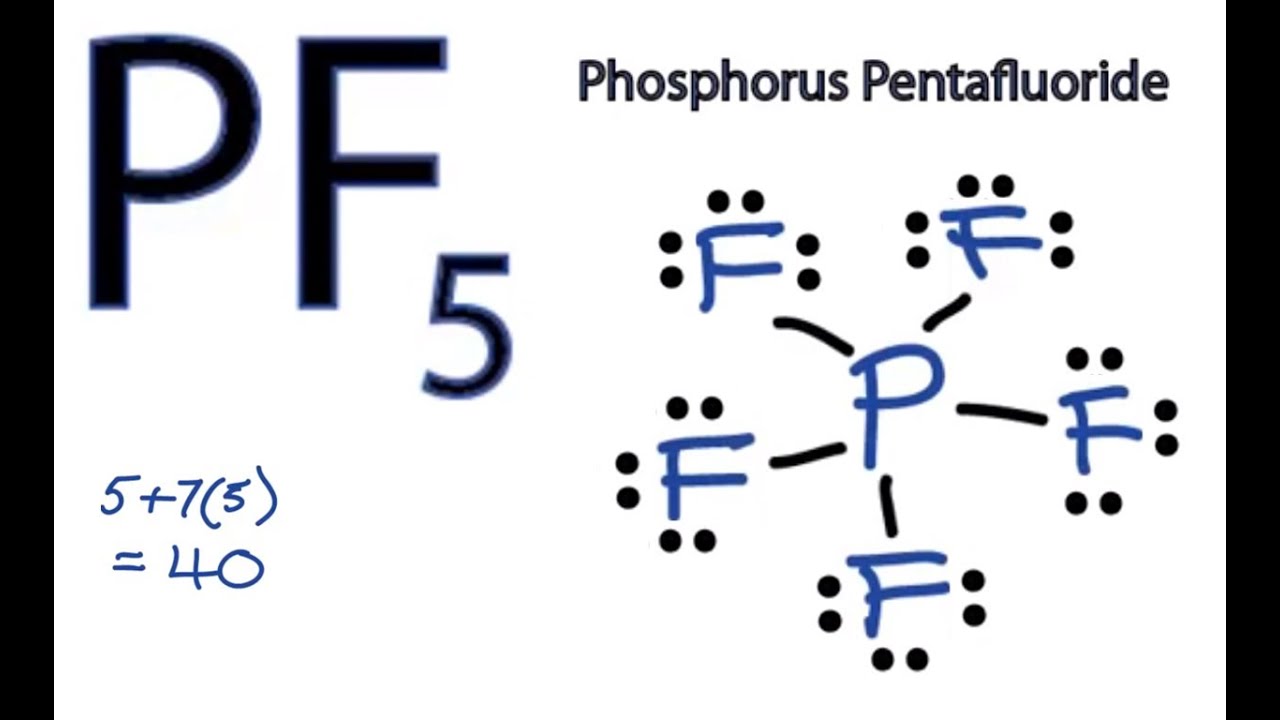
If you can do those lewis structures pcl5 will be easy. It assumes that bonds and lone pairs repel each other, and will arrange themselves to be as far from each other as possible. Lewis dot structure of atoms link. The is mainly due to the disproportionate influence or greater repulsion of the phosphorus lone pair which makes it deviate.
- Stereo Wiring Diagram For 2005 Nissan Xterra
- 2004 Acura Tl Under Hood Fuse Box Diagram
- 48re Transmission Parts Diagram
The rate of removal of pcl3 (i.e. The lewis structure of a compound can be generated by trial and error. I think thr is according to my lewis dot diagram, but i just wantd to double check. It is a lewis base:pcl3 has a lone electron pair in the highest occupied molecular orbital available for donation.( i think it's similar to the lewis structure for pcl5.
Refractive index (n), dielectric constant (εr), etc. Figure 7.12 shows the lewis structures for two hypervalent molecules, pcl5 and sf6. Looking at the pcl3 molecular geometry it is trigonal pyramidal with a bond angle of approx. Hawley's condensed chemical dictionary 14th edition.
For the pcl5 lewis structure we first count the valence electrons for the pcl5 molecule using the periodic table. Find the sum of valence electrons of all atoms in the polyatomic ion or molecule. Dots placed on 4 sides of chemical symbol. D subshell can be used for bonding.
• if it is a cation, subtract one electron for each positive charge. Which has a lewis dot structure with the greatest number of unshared pairs on the central atom? After getting the ax3n, we should look upon that table which can help us to know the molecular geometry of pcl3. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms).
Central atom usually bonded to smallest/most electronegative atoms. I also go over hybridization, shape. For this type of problem So, if you type that structure into google, you should receive the lewis structure.
Notably, when reacted with lewis acids and bases, the cyclic nature of [cl 2 p=n] 3 is generally maintained with the phosphorus centers behaving as electron acceptors and nitrogen atoms behaving. Metal complexes such as ni(pcl3)4 are known, again demonstrating the ligand properties of pcl3. These atoms belong to the exceptions in the octet rule. I know its something like this but in a bent shape.
The production rate) is equivalent to the feed rate of phosphorus and chlorine. From the lewis structure, we can use the electron pair arrangement around the central atom to predict the molecular geometry of the compound., the molecular geometry of pcl3 is trigonal pyramidal. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. However, my question is tht.if thrs a lone pair on top of the p.
Use the lewis electron structure of nh4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use. Hree pairs will be used in the chemical bonds between the p and cl. In the pcl3 lewis structure phosphorus (p) is the least electronegative so it goes in the center. Pcl3 lewis structure the compound has p and cl atoms.
Because this lewis structure has only 6 electrons around the central nitrogen, a lone pair of electrons on a terminal atom must be used to form a bonding pair. Lewis structures of h2o and so2: You can see the lewis structure of pcl3 in the practice problems below. Those lone pairs, together with the large difference in electronegativity between phosphorus pentachloride (pcl5) is an exception to the octet rule.
For the pcl3 lewis structure we first. Once we know how many valence electrons there are in pcl5 we can distribute them around the central atom and attempt to fill the outer shells of each atom. < valence shell electron pair repulsion (vsepr) theory, along with lewis structures can be used to predict molecular geometry. We start by writing symbols that contain the correct number of valence electrons for the atoms in the molecule.
I quickly take you through how to draw the lewis structure of pcl3, phosphorous trichloride. For the pcl3 lewis structure we first count the valence electron. The electronic configuration of pc13 is_ the molecular shape of pcl3 is. Looking at the pcl3 lewis.
• if it is an anion, add one electron for each negative charge. So plz help me out. A quick explanation of the molecular geometry of pcl3 including a description of the pcl3 bond angles.