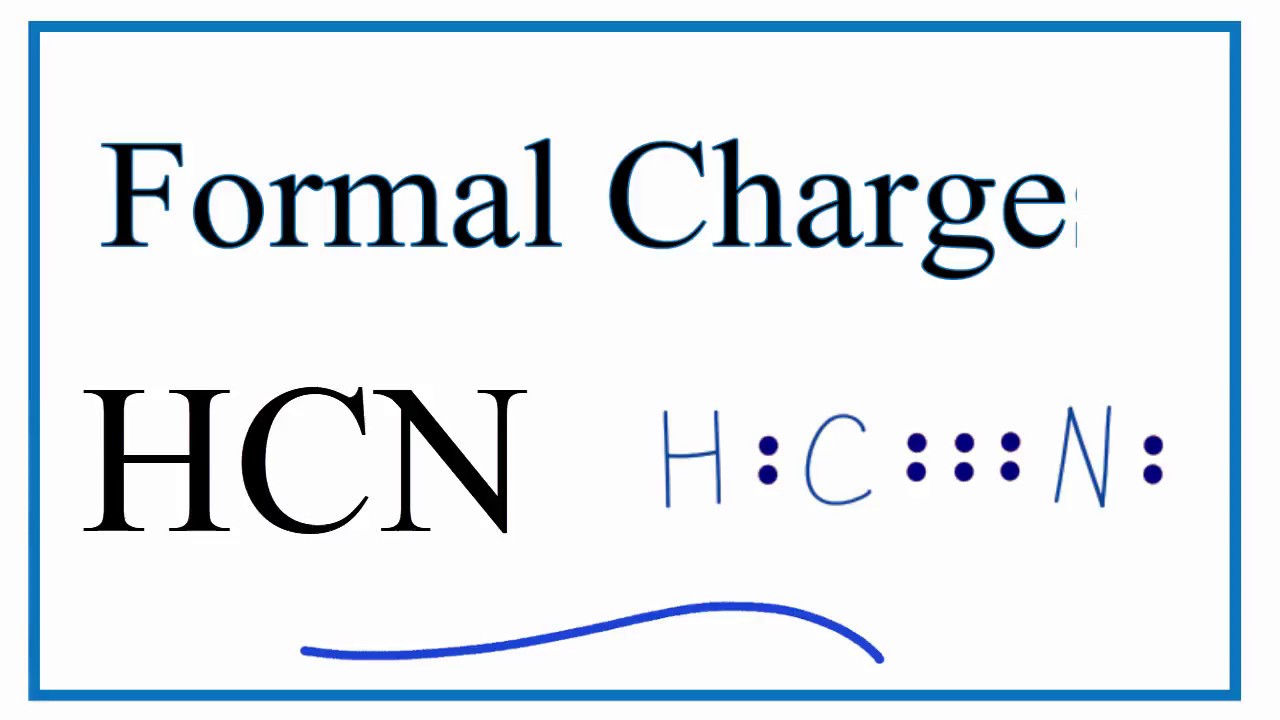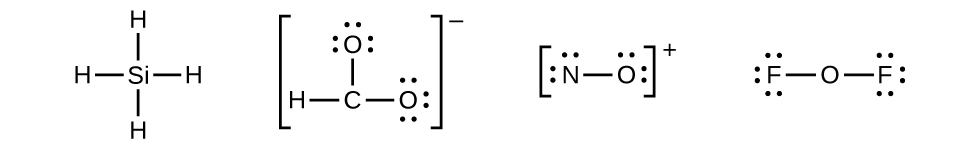Lewis Dot Structure For Hcn
These steps are easy to understand and implement.
Lewis dot structure for hcn. To draw lewis structure is extremely easy and quick. A lewis structure (also called lewis dot formulas, lewis dot structures, or electron dot structures) are pictorial diagrams that represent the bonding between atoms in a compound and the placement of electrons. It is possible to draw the structure with two electrons in place of the lines to represent the covalent bonds, which would result in there being six shared electrons between the. Draw lewis dot structures for each of the following atoms:
Draw a lewis structure for this compound, explain why you drew the lewis structure the way that you did, and predict the shape of the molecule. This example problem shows the steps to draw a structure where an atom violates the octet rule. The major reason why learning lewis dot structure is important is that it helps in predicting the number and type of bonds which can be formed around an atom. Put one electron pair in each bond 4.
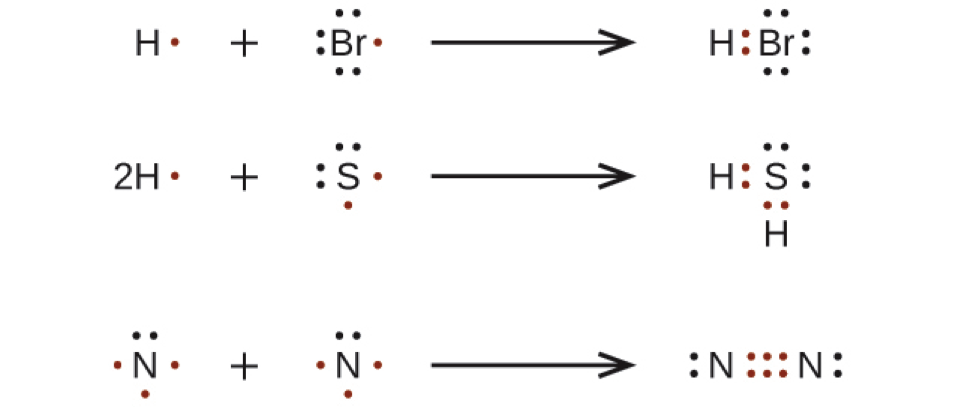
Lewis dot structures help predict molecular geometry. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Calculate the total valence electrons in the molecule. Two dots side by side represent a lone pair of electrons.
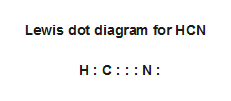
• draw lewis dot diagrams to represent valence electrons in elements and draw lewis dot structures to show covalent bonding. Lewis dot structures (or just lewis structures) were developed around 1920 by pioneering chemist gilbert lewis, as a way of picturing chemical bonding in molecules. What is the lewis structure of hcn? Drawing lewis dot structures (also known as lewis structures or lewis diagrams) can be confusing, particularly for a beginning chemistry student.
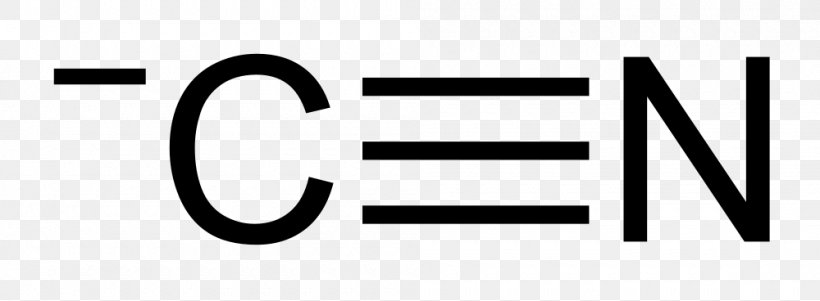
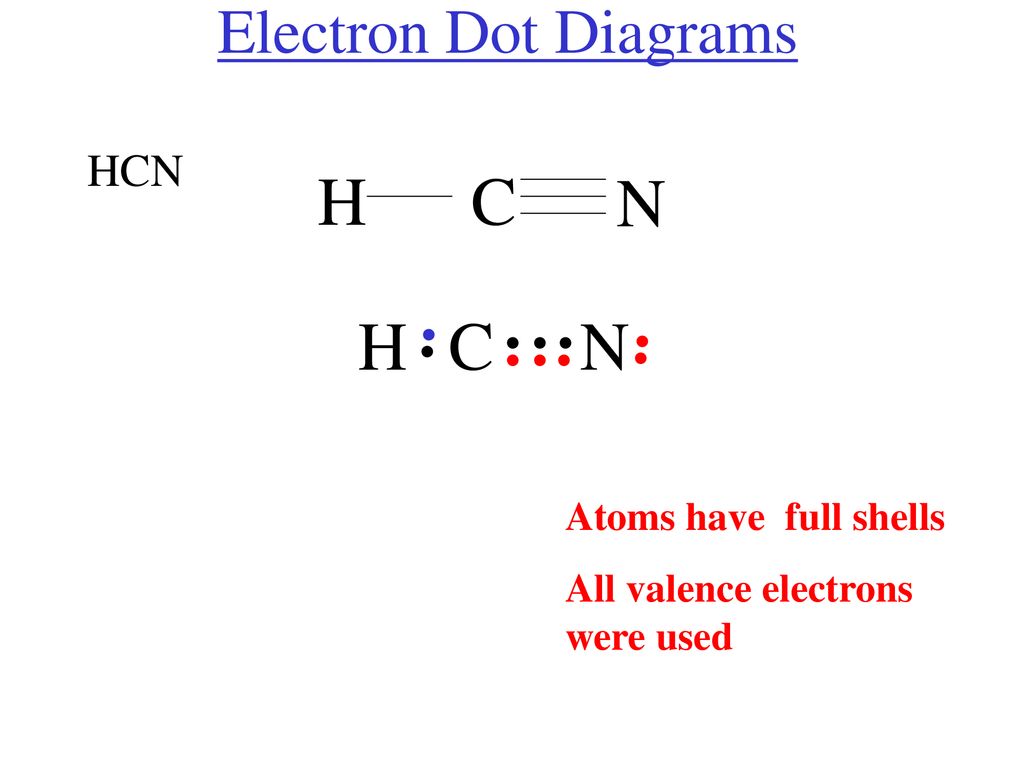
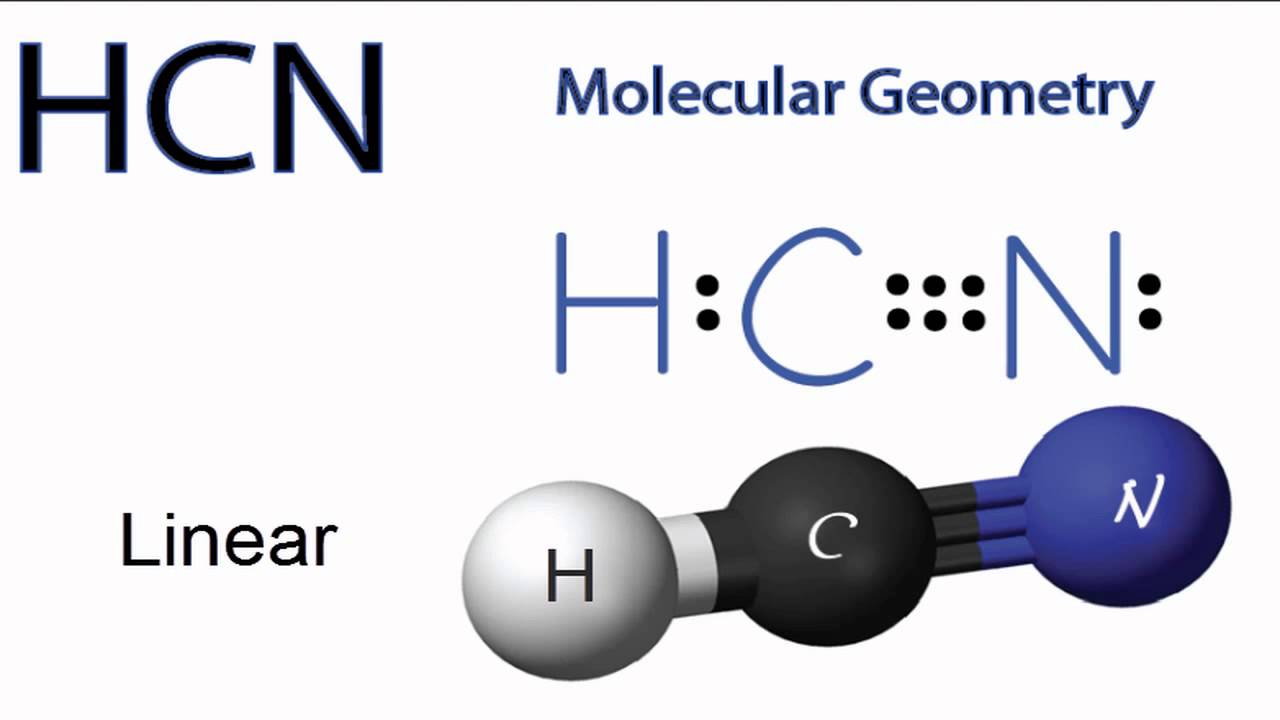
The molecule hcn has one of the most fascinating lewis structures. Results for lewis dot structure for hcn. What are lone pairs and how are they represented in a lewis dot diagram? So nitrogen (n) would look like this
Lewis dot structures can be drawn for a variety of molecules based on a few simple steps. Basic lds's are covered in section 9.5 and 9.7 in tro. How to draw a lewis structure (octet rule exception). The lewis structure, or lewis dot diagram, shows the bonding between atoms of a molecule and any electrons that may exist.
Organic chemistry lewis structures and bonding lewis dot diagram. N = 8 (s) + 3 x 8 (o) = 32 ( reso there are three possible structures for so3. To draw lewis structure is extremely easy and quick. The lewis symbol for an atom depicts its valence electrons as dots around the symbol for the element.
Download lewis dot structure for hcn for free. Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. How is the total number of electrons represented in a lewis structure determined? A simple procedure for drawing lewis dot structures was given in a previous post entitled lewis structures and the octet rule.
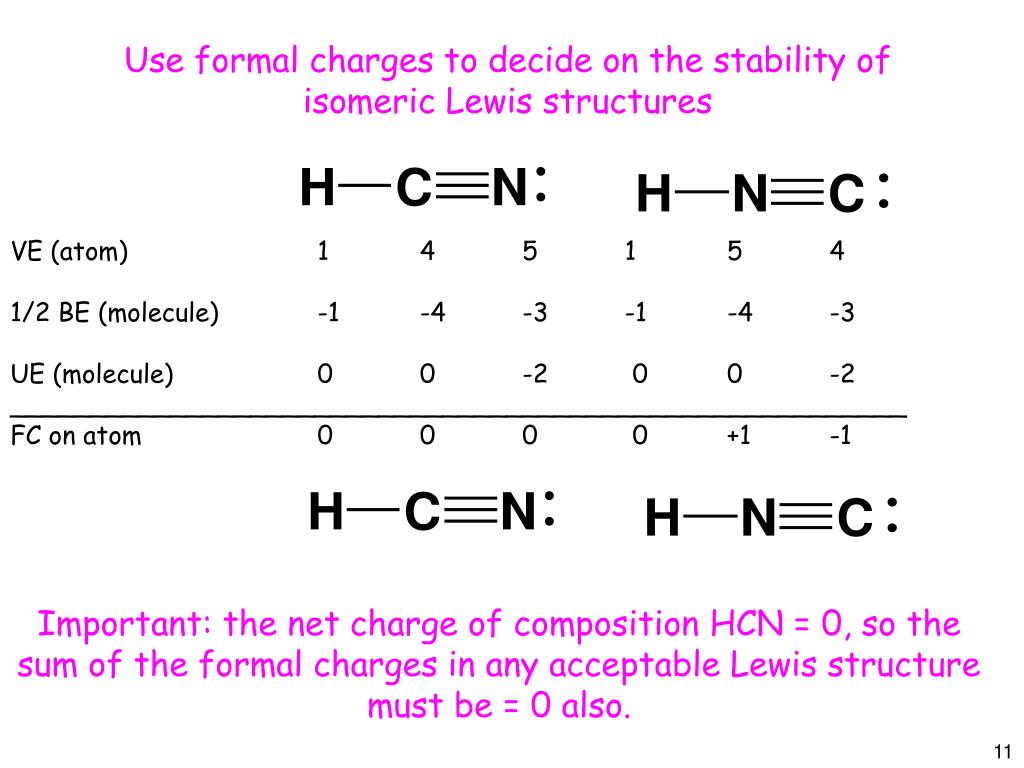
Draw lewis structures for the following •. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. It is one of the few which features a nitrogen triple bond.
Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. Br3o3 co2 po43so42clf3 hcn note: The lewis structure for li is li with one dot to the right of the element. Hydrogen cyanide (hcn) is a colorless gas.
Put least electronegative atom in centre 3. Lewis structures, part 2 of 3 practice drawing lewis structures. Chemistry q&a library what is hcn lewis dot structure. • use valence shell electron pair repulsion (vsepr) model to draw and name molecular shapes (bent, linear, trigonal planar, tetrahedral, and trigonal pyramidal).
The structure on the right is the lewis electron structure, or lewis structure, for h2o. The nitrogen is having a full octet by having eight electrons in total. However, these structures are helpful in understanding the bonding and valence electron. Lewis structures were first introduced in 1916 by gilbert lewis and have been adopted as.
In lewis dot structures each dot represents an electron. Alternatively a dot method can be used to draw the hcn lewis structure. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Learn about an exception to the octet rule:
It is poisonous and smells like almonds. Lewis structures (also known as lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. All formats available for pc, mac, ebook readers and other mobile devices. Determine the common oxidation number (charge) for each of the following ions, and then draw their lewis dot structure.
Each dot represents one electron. First of all, refer to the periodic table and count the electrons by matching the columns. The lewis structure (lewis dot diagram) for hcn. As hcn has ten valence electrons for the lewis structure, now there are no electrons left.
Lewis structure is very important in chemistry, because they are used in many important concepts of general chemistry such as chemical bonding, resonance, valence shell we can learn to make accurate lewis dot structures in 4 simple steps. Lewis symbols of the main group elements. First of all, refer to the periodic table and count the electrons by matching the columns. Lewis structure is basically a graphic representation of the electron distribution around an atom.