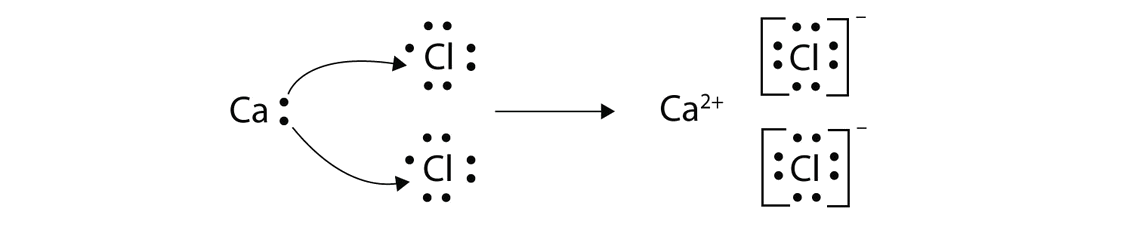
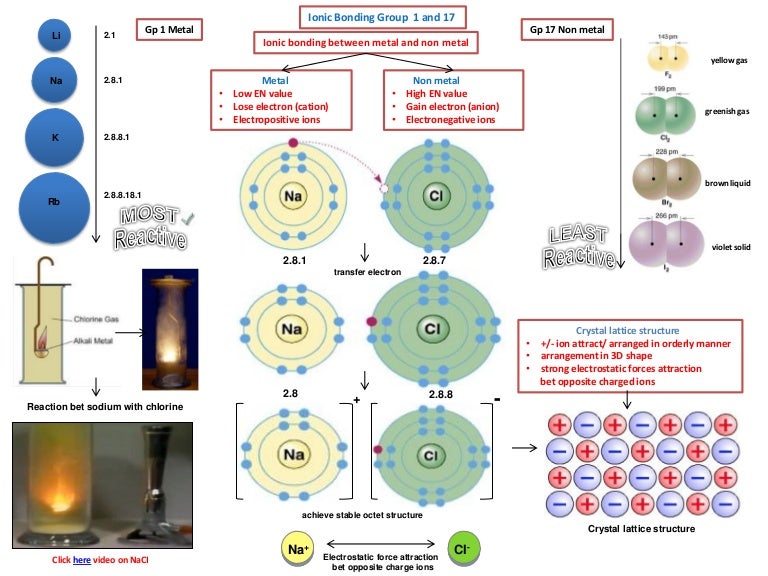
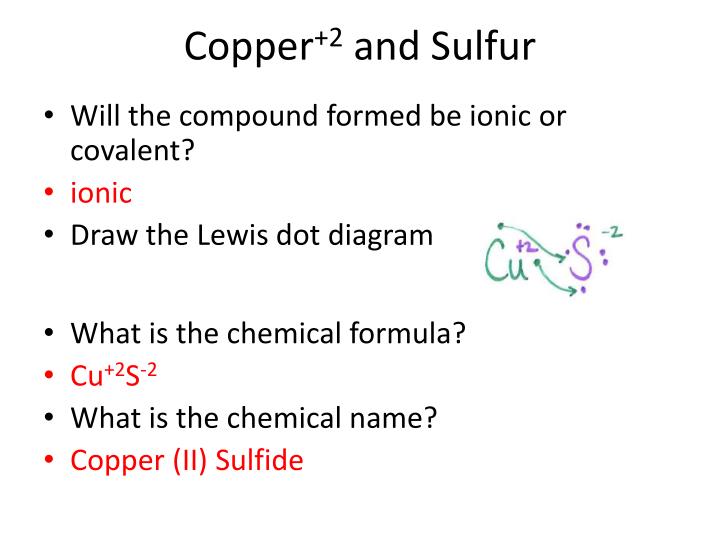
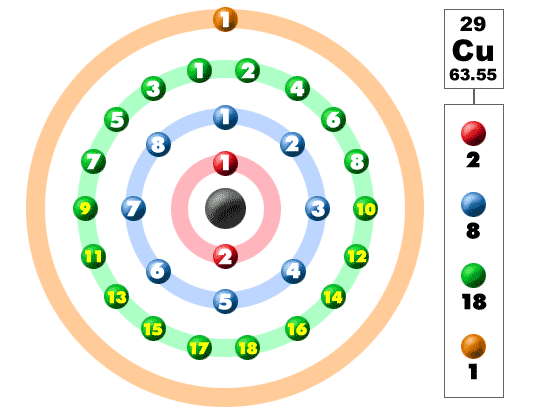
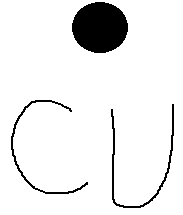
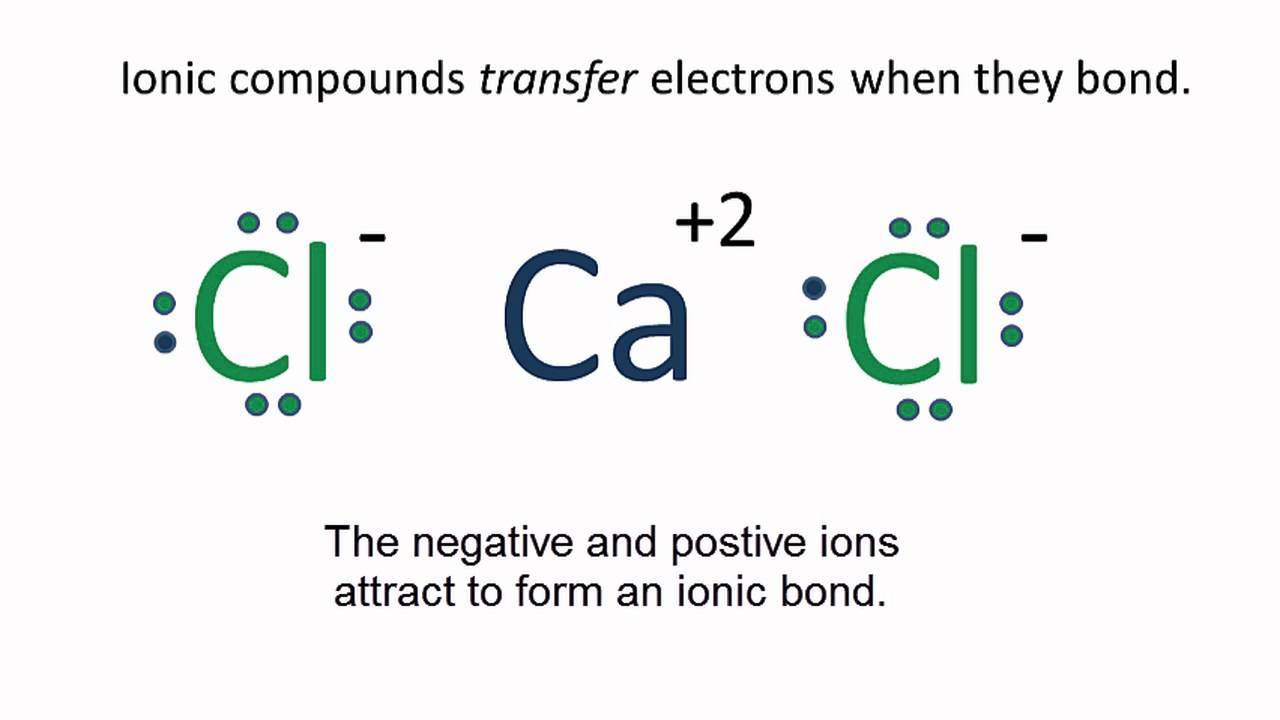Lewis Dot Diagram For Copper
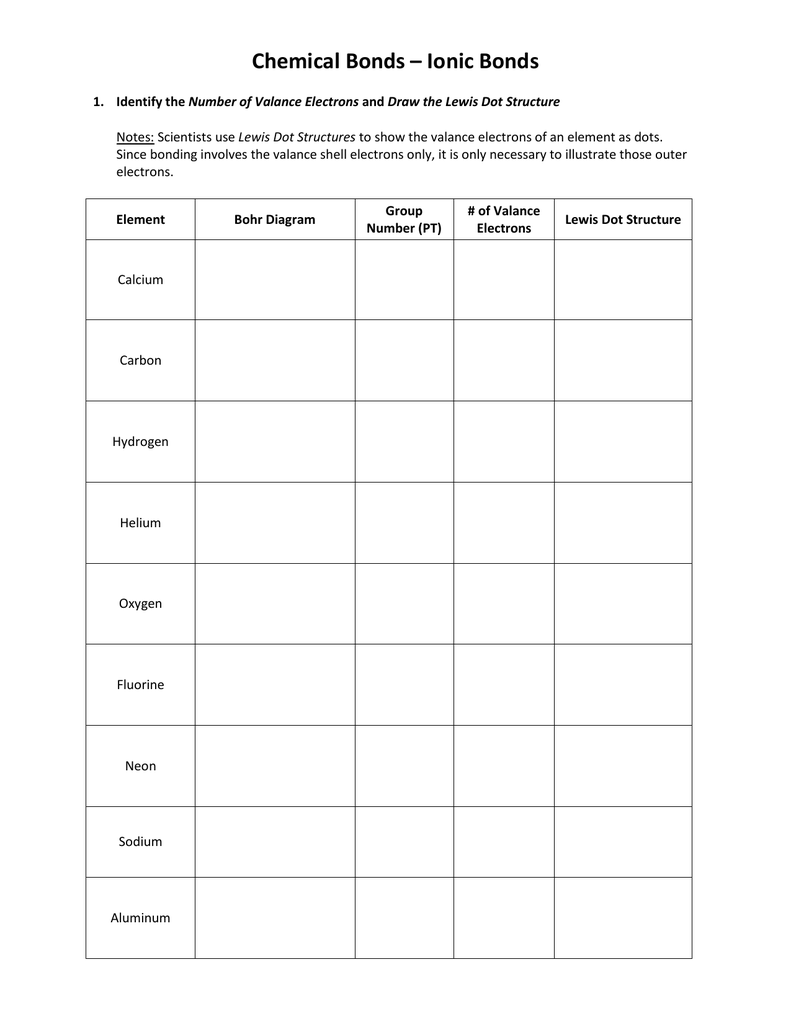
By the end of this section, you will be able to a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of.
Lewis dot diagram for copper. I need to know how to draw lewis dot diagrams for compound containing polyatomic ions. So, its bond will be notated in a lewis diagram as 3 parallel lines. How do you draw a lewis dot diagram for copper(ii) sulfate? Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule.
Copper wants to donate 2 electrons for a full valence shell (see it's column in the periodic table). Using lewis dot structures and the octet rule, we can predict and represent the electronic structure of covalently bonded molecules. Draw skeletal structure of compound showing what atoms are bonded to each other. How to determine the lewis dot structure of copper ii.
Introduction to lewis structures for covalent molecules. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. We will start by constructing the lewis dot diagram for the molecular compound known as water, hzo. A lewis structure is a diagram that shows how valence electrons within a compound are distributed among its atoms.
Lewis, who described them in a 1916 article titled, the atom and the you can draw a lewis dot structure for any covalent molecule or coordination compound. The lewis structure contains the element symbol with dots representing electrons. Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. The method explored in this lesson will be a visual representation of the valence electrons.
How to draw lewis dot structures. We call that a lone pair. Two dots at top (or on either side, or on the bottom) could represent any element with two valence electrons. The lewis structure, or lewis dot diagram, shows the bonding between atoms of a molecule and any electrons that may exist.
Drawing lewis dot structures (also known as lewis structures or lewis diagrams) can be confusing, particularly for a beginning chemistry student. While the lewis diagram correctly predict that there is a double bond between o atoms, it incorrectly predicts that all the valence electrons are paired (i.e., it predicts that. Organic chemistry lewis structures and bonding lewis dot diagram. What is the lewis dot diagram for copper?
Lewis dot structures (or just lewis structures) were developed around 1920 by pioneering chemist gilbert lewis, as a way of picturing chemical bonding in molecules. But i have copper, and i cant find the lewis dot diagram for that anywhere! Elements for lewis dot diagrams. For example, n2 (nitrogen gas) has a triple bond connecting the 2 nitrogen atoms.
How to draw lewis diagrams for compounds. Diagram showing how copper immersed in copper 2 sulphate can be measured. Draw a lewis electron dot diagram for an atom or a monatomic ion. Lewis dot diagrams of selected elements.
It occupies its own stable orbital, as shown in the stick diagram on the right. Use the bohr models to determine the number of valance electrons. Elements in the same column of the periodic table. How is the total number of electrons represented in a lewis structure determined?
This chapter will explore yet another shorthand method of representing the valence electrons. Since you can only have 8 dots, 2 on. Before we begin, let s refresh our memory of what the lewis dot. A lewis dot structure is like a simplified electron.
There is no lewis structure for sodium iodide it is an ionic bond so therefore requires an electron dot diagram. Lewis structures are a useful way to summarize. Electrons in the lewis structure (electron dot diagram) are paired to show the bonding pair of electrons. Lewis structures, also known as electron dot structures, are named after gilbert n.
Those electrons that are shared by two those electons that are located on a single atom are referred to as lone pairs and represented by two dots. 512 x 411 jpeg 38 кб. Lewis dot diagram lewis dot diagram. 6.1 lewis electron dot diagrams.
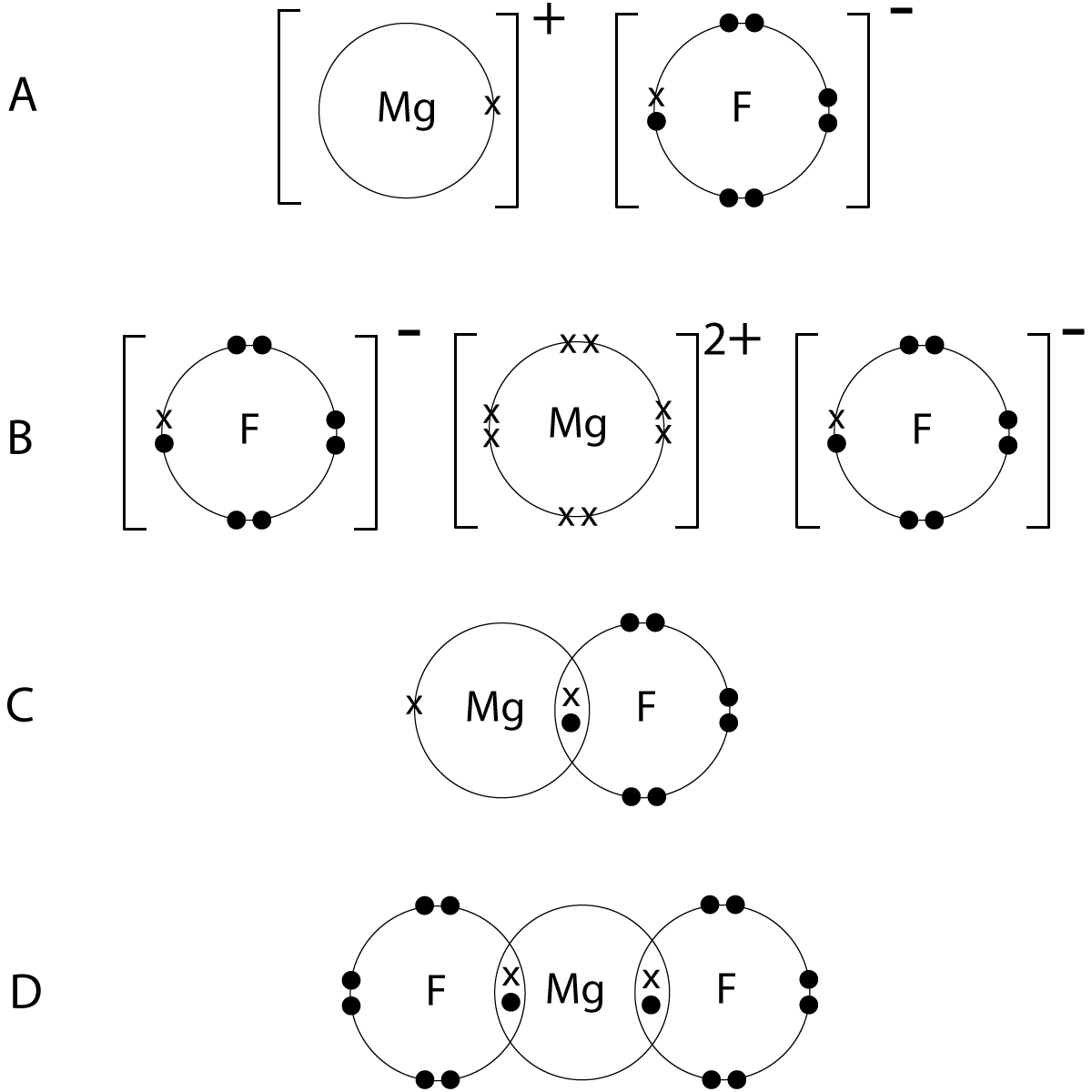
Lewis dot diagrams are a tool for predicting the most likely connectivity, or arrangement of bonds between atoms, in a molecule. Diagraming elements of molecular structure. _____ lewis dot practice drawing lewis structures: Lewis dot structures of ionic and covalent compounds the following lesson looks at drawing electron dot or lewis dot diagrams of various ionic lewis structures, part 1 of 3 learn a simple process for drawing correct lewis diagrams, and practice drawing lewis structures that obey the octet rule.
Students will be able to interpret and draw lewis dot diagrams for individual atoms and both covalent and ionic compounds. Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom. In almost all cases, chemical bonds are formed by interactions of valence electrons for example, the electron dot diagram for iron (valence shell configuration 4s23d6) is as follows: Draw a lewis electron dot diagram for an atom or a monatomic ion.
How to make copper nitrate solution. 1280 x 720 jpeg 26 кб. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. What is the lewis dot diagram for copper socratic.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.