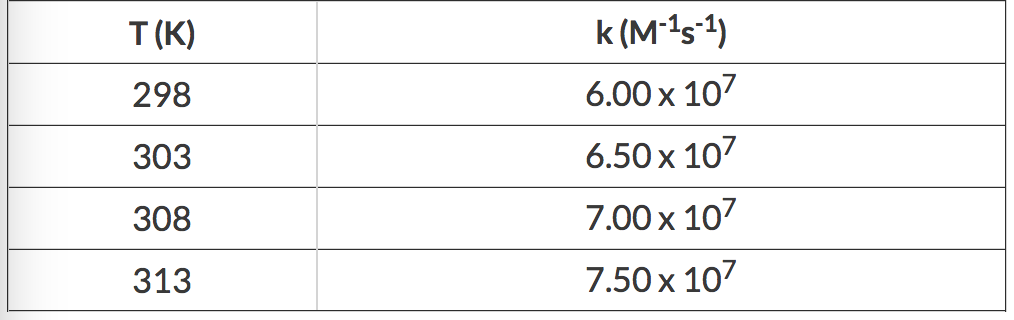
Construct An Orbital Diagram To Show The Electron Configuration For A Neutral Magnesium Atom Mg
Electron configuration shows the arrangement of electrons in an atom.
Construct an orbital diagram to show the electron configuration for a neutral magnesium atom mg. Each box represents an orbital. Convert from orbital representation diagrams to electron configuration codes. This chart is straightforward to construct. Chromium is a transition metal and it has 24 electrons and here is the orbital diagram.
An atom needs to have 8 electrons in its outer shell in order to feel complete. 1s2 2s2 2p6 3s0 this is the electronic configuration of magnesium cation. Each set of orbitals, when full. Use the buttons at the top of the tool to add sublevels.
Electron configuration can be designated using a shorthand notation of the general form nl# (e.g from left to right across a period. The basis of this prediction is a rule known as the aufbau the energy of an orbital depends on both its size and its shape because the electron spends more of its time further from the nucleus of the atom. If we're going to make this short hand and make the electron configuration for this we would make this 1s2, 2s2. An electron configuration shows the distribution of electrons of an atom or a molecule.
- Schumacher Se 82 6 Wiring Diagram
- Yard Machine 42 Inch Deck Belt Diagram
- Dayton Capacitor Start Motor Wiring Diagram
Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg. It is the second element in group 3, so 3p is the next orbital that is filled. 1s^2,2s^2,2p^6 is the electronic configuration of mg^(+2). The ion's configuration would therefore be:
Electron configuration electron configuration is shorthand for the arrangement of electrons in errata: In the neutral atom it has an electron configuration of 1s2 2s2 2p6 3s2 and the orbital diagram is ↑↓ ↑↓ ↑↓↑↓↑↓ ↑↓. How many electrons are in a these levels of organization are shown by the boxes of the gizmo. In writing the electron configuration for calcium the first two electrons will go in the 1s orbital.
The electron configuration for cl should be [ne]3s23p5. The simplest atom hydrogen has 1 electron. The arrangement of electrons within an atom is called the electronic configuration and the in simple terms, it means that every time, electrons are first filled up in an orbital singly and only when for a given electron configuration, the lowest energy term is the one with the greatest value of spin. We need to write the electron configuration for an.
• boxes or lines represent each orbital • arrows within boxes represent the electrons • max two per box • opposite direction (represents opposite spin) section 3.5. A magnesium atom has 12. Orbital notation electron configuration notation noble gas notation follow along write the electron configurations for the following atoms: The electron configuration of a neutral magnesium atom is:
Determine the electron configuration for elements and ions, identifying the relation between electron shells and subshells. How to write the electron configuration for calcium (ca). Electron configurations are written so as to clearly display the number of electrons in the atom as well as the number of electrons in each for instance, if we want to write an electron configuration for an uncharged calcium atom, we'll begin by finding its atomic number on the periodic table. 20 practice problems write the electron configuration and the orbital diagram for magnesium.
An atom's electrons exist in discrete atomic orbitals, and the atom's electron configuration can be determined using a set of guidelines. Start filling the question 1 write the electronic configuration for the following atomic numbers (a) 23 (b) 30 (c) 19. A neutral atom has the same number of electrons and protons. Electron configuration notation simplifies the indication of where electrons are located in a specific atom.
This page shows the electron configurations of the neutral gaseous atoms in their ground states. The element in question is magnesium (mg) with atomic number 12. Write the electron configuration for nitrogen. An orbital is a potential space for an electron.
After clicking check, note the electron. Electron configurations describe where electrons are located around the nucleus of an atom. Video explanation on the exceptions to electron configuration. 1s2 2s2 2p6, which is isoelectronic with.
Alright so let's talk about chromium. Electronic configuration or electron configuration is the arrangement of electrons in different orbitals the atomic number in neutral atom represent the number of electrons 2. Orbital diagrams orbital diagrams are pictorial descriptions of the electrons in an atom. This need is what each sublevel contains a certain number of orbitals.
In writing the electron configuration for magnesium the first two electrons will go in the 1s in order to write the mg electron configuration we first need to know the number of electrons for the mg in all neutral atoms both protons and electrons are numerically equal. Simply make a column for all the s orbitals with each n shell on a separate row. 1s2 2s2 2p6 3s2 or in shorthand [ne] 3s2. Click within an orbital to add electrons.
Click within an orbital to add electrons. Create a proper electron configuration for sodium. So instead of just having one electron in. Kbr, lif, and nacl 2 electrons:
The superscripts are the number of electrons in the orbitals. For each atom the subshells are given first in concise form, then with all subshells written out. Which nonmetals could form an ionic compound with magnesium with the formula mgx2 (where x represents the nonmetal)? The valence electron are those in the 3s subshell and these are lost to create an ion of mg2+ that is isoelectronic.
There is a specific notation that can quickly show you where the what is an atomic orbital? 3 orbital diagram another way of writing the electron configuration. It doesn't save much space here, but imagine if you were writing the electron configuration for say well, a neutral helium atom is going to have two electrons. It will go into the 1s orbital with a spin in either this can be seen on the orbital filling diagram, but not on the electron configuration which provides less the table below shows the electron configuration for the first 20 elements on the periodic table.
Draw the electronic configuration for potassium using. As an atom gains electrons, they fill different orbitals sets according to a specific order. Distinguish between outer energy level (valence) electrons and let's begin this section with the orbital box (or the orbital representation diagram) for a neutral atom. Magnesium is in group 3, so it already has 1s, 2s, and 2p filled.