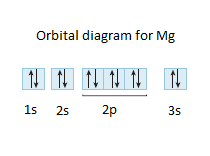
Construct An Orbital Diagram For Mg
Determine the irreducible representation of the orbitals of the central atoms.
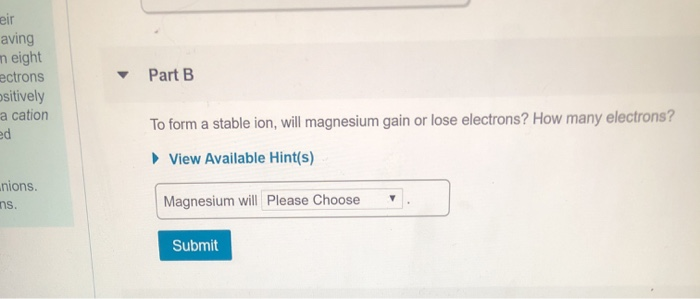
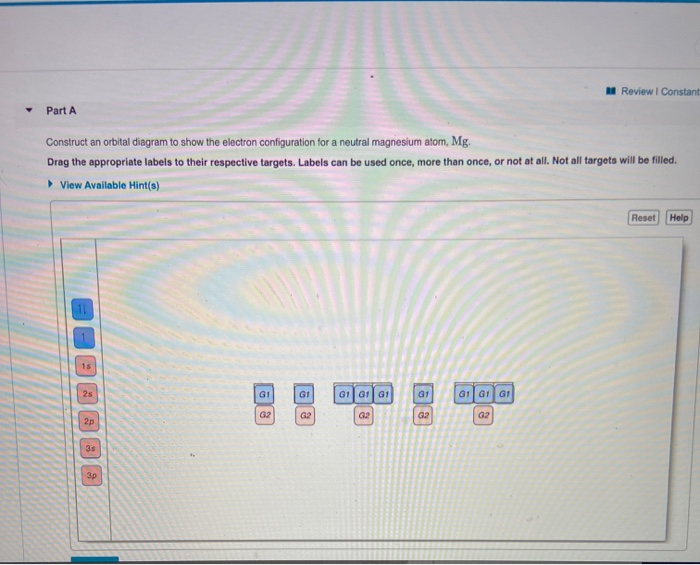
Construct an orbital diagram for mg. Use vsepr theory to classify and determine the geometry around each central atom. List the known quantities and plan the problem. In an orbital filling diagram, the figure 2. Draw the lewis structure for the molecule.
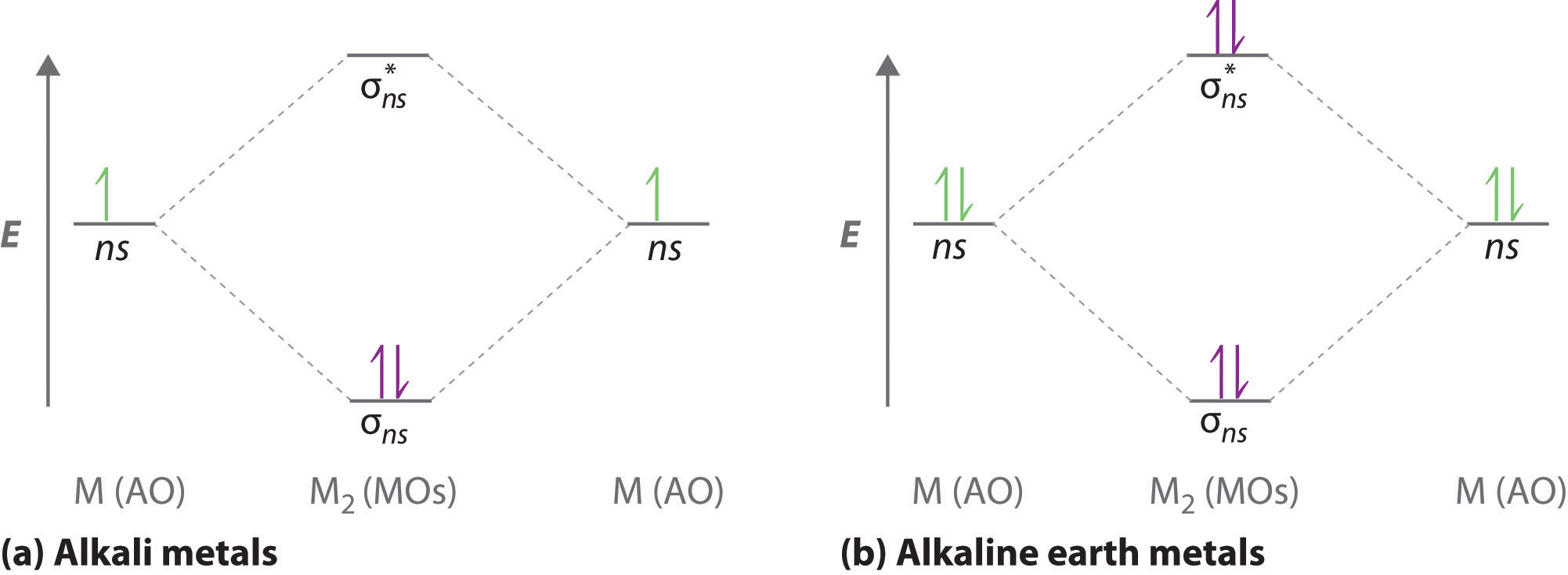
We represent this configuration by a molecular orbital energy diagram (figure 9) in which a single upward arrow indicates one electron in an orbital, and. Drag the appropriate labels to their respective targets. Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. In order to figure out where electrons go in an atom we have to follow 3 main rules.
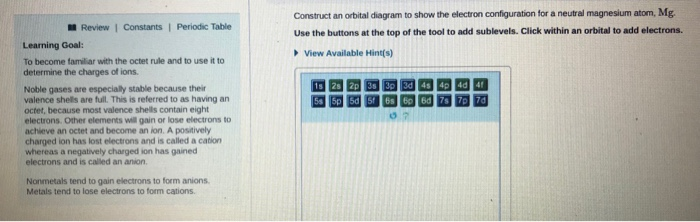
(b) f 4 sp hybridized orbital xe 5s 5p (c) (note: What orbital overlaps are involved in the n─h bonds? Symmetry of the central oxygen's orbitals. Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg.
- 2015 Freightliner Cascadia Fuse Box Diagram
- 2005 Honda Odyssey Fuse Box Diagram
- 6 Volt Positive Ground Alternator Wiring Diagram
First, determine the point group of the molecule. Students also viewed these organic chemistry questions. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration.
Draw the orbital filling diagram for carbon and write its electron configuration. Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg. According to the aufbau process, sublevels and orbitals are filled with electrons in. Based on these values, what type of bond is expected for a compound formed between mg and o?
Dublin schools lesson orbital diagrams and electron configurations. This article explains how to create molecular orbital diagrams in latex by means of the package modiagram. Alright let's talk about orbital diagrams. In reality, the 5d orbitals of xe is involved and the homo has d character) 12.
Label the atoms with their appropriate letters to the left of each electron configuration and number notations beneath the filled text boxes to complete your orbital box diagram. A partial orbital diagram shows only the highest energy sublevels being filled. Draw an orbital diagram for beryllium (z=4). Here are the steps for constructing a hybrid orbital diagram for ethylene.
Chemistry q&a library construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg. This is the molecular orbital diagram for the homonuclear diatomic be2+, showing the molecular orbitals of the valence shell only. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Orbital diagrams are a pictorial description of electrons in an atom.
Draw out the possible bonding interaction of the orbitals from the peripheral atoms. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. Each carbon atom is an ax₃ system, so the geometry is trigonal planar. Given several orbitals at the same energy level, electrons will enter each orbital first, then add a second electron to an orbital (singles then doubles).
Draw out the orbitals of the central atoms. Valence because the electronegativity of the two atoms are unequal, the molecular orbital diagram will no longer be symmetric. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Reality check to construct an orbital diagram, start with the electron configuration and apply hund s rule.
13 qualitative molecular orbital energy level diagram of the dimer d orbitals with the 14 electrons showing the electronic configuration 525 27i47i 4o2 (singlet) and the first excited state 828. They consist of the symbol for the element in the. Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons. The first major step is understanding the difference between two major theories:
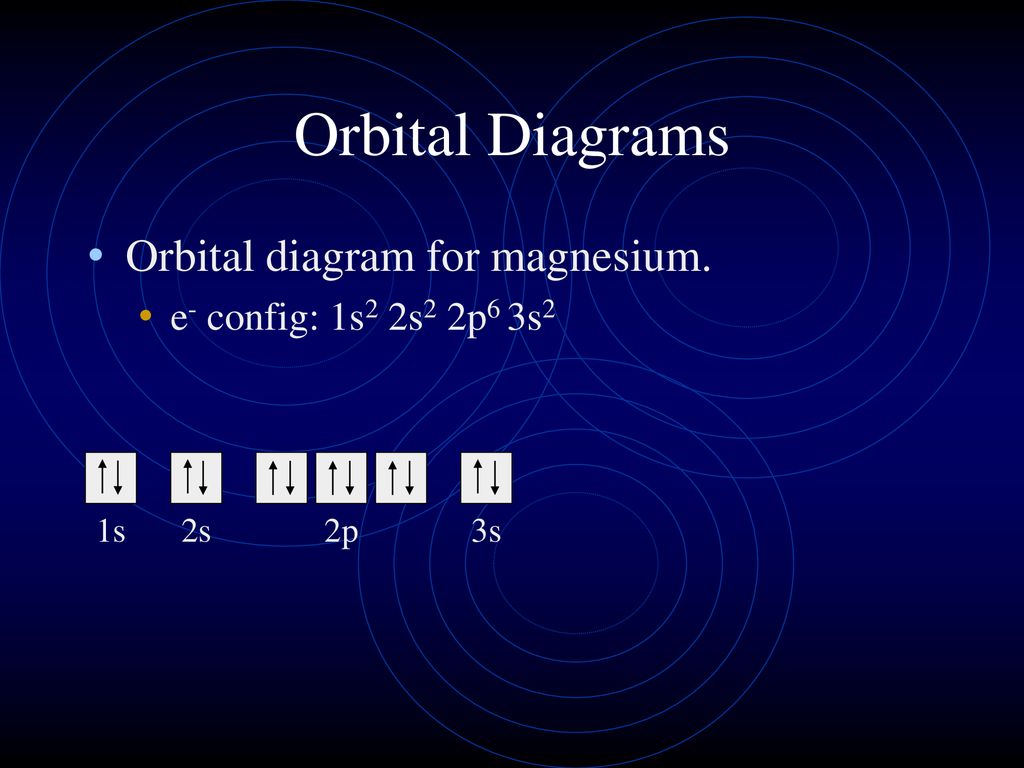
This problem has been solved! Magnesium (mg) is atomic number 12, so it has 12 electrons. Quantum numbers and electron configurations what is the electron configuration and orbital diagram for a phosphorus atom? Orbital diagrams & electron configurations for atoms and ions orbital diagrams represent the arrangement of electrons in orbitals.
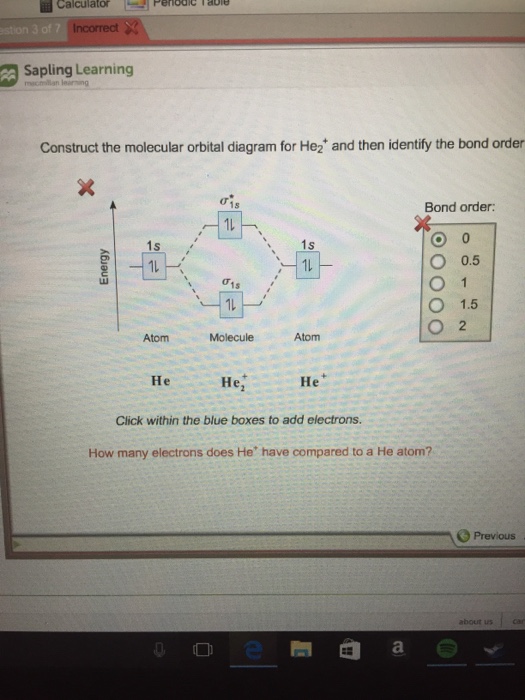
Click within an orbital to add electrons. Partial orbital diagrams and condensed configurations. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the aufbau principle to order the orbitals and hence. 1s bond order o 0.5 o 1.5 1s ã€ã€ 1s 1ー 1s 2 atom molecule atom 2+ he he he click within the blue boxes to add electrons.
The first one being the auf bau principle, the auf bau principle states that each electron occupies the lowest. The electronegativity value for mg is 1.2 and the value for o is 3.5. What is the hybridization at each nitrogen of. Construct an orbital diagram like that in what kind of orbital is the unshared pair?
(a) construct a molecular orbital energy level diagram for po + [ignore s, p mixing]. In order to construct an mo diagram for water, we'll take a stepwise approach: Construct the orbital diagram for as? Orbital filling diagrams for hydrogen, helium, and lithium.
What is the orbital diagram of … Question 3 of 16 map& mapoob sapling leaning construct the molecular orbital diagram for he and then identify the bond order. • boxes or lines orbital filling order for elements beyond period 2 ….corresponds to atom's location in periodic table! Only 2 electrons per orbital, they must have opposite spin.
It explains how to write the orbital diagram. Orbital box diagrams can be easily constructed in microsoft word with text boxes. Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy dot diagrams are very different to orbital diagrams, but they're still very easy to understand. First, determine the way in which the hydrogen atoms can combine (in phase or only orbital of correct symmetry are able to combine.
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down refer to the related link to see an illustration of an orbital diagram for aluminum. This is where group theory becomes useful.