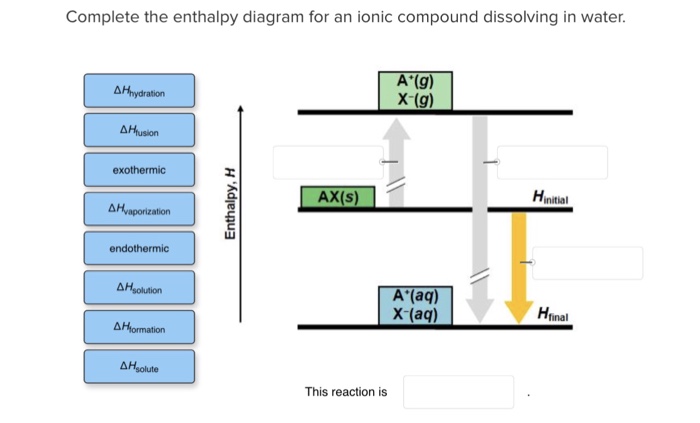
Complete The Enthalpy Diagram For An Ionic Compound Dissolving In Water
A familiar example of an ionic compound is table salt or sodium chloride.
Complete the enthalpy diagram for an ionic compound dissolving in water. As a result, the solution becomes an electrolyte. Solubility rules for ionic compounds in. As an example, let's consider what happens to an ionic compound, such as. Learn vocabulary, terms and more with flashcards, games and other study tools.
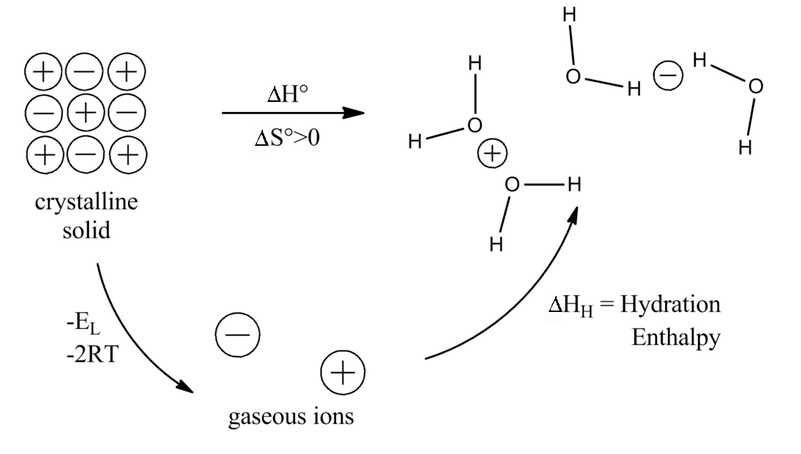
For example, when the soluble compound sodium carbonate dissolves in water, the partial negatively charged side of the polar water molecules surround the positively charged sodium ions. Below is a figure of a small crystal of sodium chloride surrounded by water molecules. Generally speaking, water is good at dissolving ions and polar molecules, but poor at dissolving nonpolar molecules. For dissolution of ionic compounds, if the hydration enthalpy is more than lattice enthalpy, ample amount of energy will be available to saparate more ions hydration enthalpy is energy released when separated ions are dissolved in water.
All ionic compounds that dissolve behave this way. Only ionic compounds which are soluble in water (forming aqueous solution) will dissociate into ions in water. Solubility rules for ionic compounds use the solubility rules in your textbook or the ones from lecture to complete question 1. The following diagram shows how to write the ionic equation for the reaction of aqueous sodium carbonate with aqueous barium.
To determine if an ionic compound is soluble i.e., will dissolve in water, we use the solubility rules: When a covalent compound dissolves in water, it does not dissociate into ions. Elements or molecules which are positively charged are called cations and elements or molecules which what happens when an ionic compound dissolves in water? The teacher stated that the ionic compounds dissolve in water except some carbonates.
An ionic compound is made up of charged particles, called ions. Salt has a high melting point of 800ºc. To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. Ionic compounds are composed of ions;
Water molecules separate the ions in ionic compounds and draw them into solution. The enthalpy change when one mole of an ionic compound is dissolved in water to produce aqueous ions. Lattice enthalpy δhle(compound) is defined as the energy released when 1 mole of a solid ionic is formed from its separated constituent gaseous ions (an exothermic for diagram simplicity, each ion is surrounded by four water molecules, though in reality, much more than this ñ see later. Others generally do not dissolve in water and are considered insoluble.
It has a giant lattice structure with strong electrostatic forces of attraction. The enthalpy change when one mole of an ionic solid dissolves in an amount of water large enough that the dissolved ions are well separated and do not interact with each other. This is why solid ionic compounds form crystals with regular shapes. The lattice arrangement continues in three dimensions.
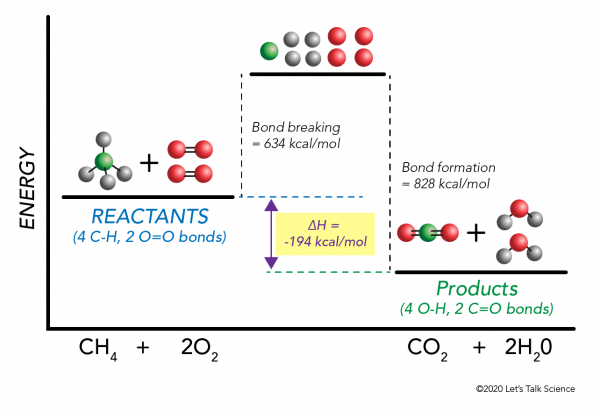
The enthalpy change when one mole of a substance undergoes complete combustion in oxygen. While a salt crystal is an electric insulator, saline. I show students that a water molecule looks like a mickey mouse head (if they don't. (this behaviour was first suggested by the swedish write the complete ionic equation for each chemical reaction.
Water will pull the cations and anions apart. When these ions are removed, the ionic equation is called the net ionic equation. This video covers, how to predict products, how to balance a chemical equation, how to identify the solubility of a compound, how to write a complete ionic. Insoluble substance cannot dissociate into ions in water.
Because there are no free electrons or ions in the water (electrolytes) dissolved covalent in contrast, salt (nacl) is an ionic compound. Sodium chloride is an ionic compound with a positive sodium ion (na+) and a negative chloride ion. Increasing the temperature of the water allows this process to happen quicker while decreasing the temperature causes the process to slow. Since dissolving depends on the interaction between water and the substance being dissolved students will be able to explain why different substances dissolve to different extents in water.
How many ions arc obtained from each formula unit when the compound is dissolved in water? Sodium chloride (nacl), and student name: Osmotic pressure of a solution of a compound is given and number of ions in the solute compound has to be determined. Start studying dissolving ionic solids (thermodynamics).
When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. First of all, the distinction between the hydration energy of gaseous ions, which is the enthalpy change when gaseous ions dissolve in sufficient water to give an infinitely dilute. When an ionic compound is dissolved in water it will conduct electricity better. When an ionic compound dissociates in water, water molecules surround each ion and separate it from the rest of the solid.
After diagramming an ionic compound, complete with alternating ions with charges indicated, i use a different colored marker to outline the atoms in the lewis dot structure of water previously drawn on the whiteboard. One source to use is listed below If an ionic compound dissolves in water, it separates into individual charged ions. Hesss law here is an example of using hesss cycle for dissolving an ionic compound:
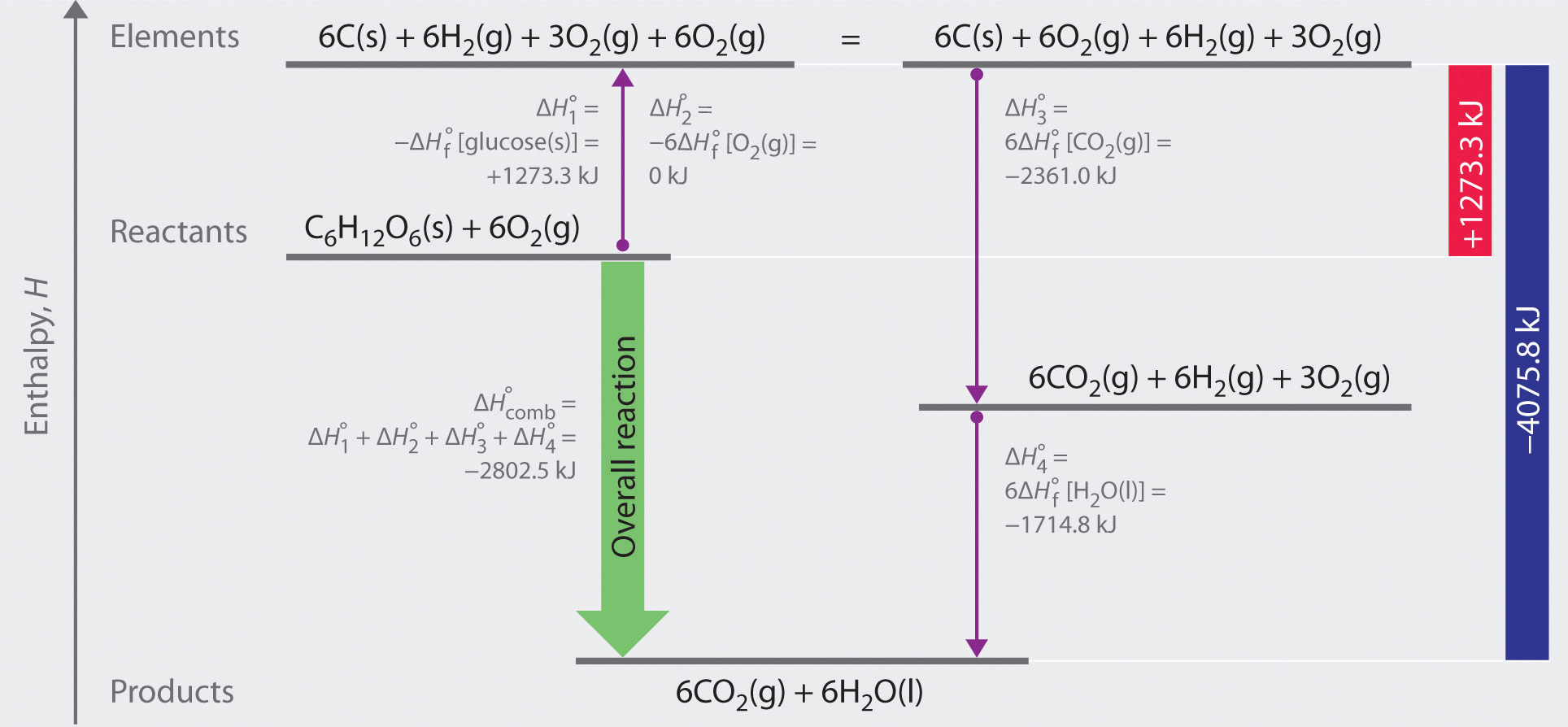
Solubility rules many ionic compounds are soluble i.e., they dissolve in water. When ionic compounds are dissolved in water the dissociated ions are free to conduct electric charge through the solution. Transcribed image text from this question. The standard enthalpy of formation refers to the enthalpy change when one mole of a compound is formed from its elements.
(a polar molecule is one how does the formation of a hydration shell cause a solute to dissolve? Lead (ii) nitrate, pb (no₃) ₂ is a solution (ion compound) which, if dissolved in water. There are only two stages involved with this process the diagram on the left shows a lattice of positive and negative ions held together by strong electrostatic forces. Enthalpy changes associated with the addition of water molecules to the li+ and cl− in the gas 7.
Sodium (na) and chlorine (cl) have very different electronegativity values, so when. Enthalpy changes in solution ionic compounds. Most ionic compounds are soluble in water because the electrostatic forces of the polar water molecules are stronger than the electrostatic forces there are several exceptions, however, where the electrostatic forces between the ions in an ionic compound are strong enough that the water. Thus breaking of ionic bond requires energy while.
Solubility rules name chem worksheet 151 cations many ionic compounds will dissolve in water so we say they are soluble. The heat association with dissolving a particular solute in a particular solvent.