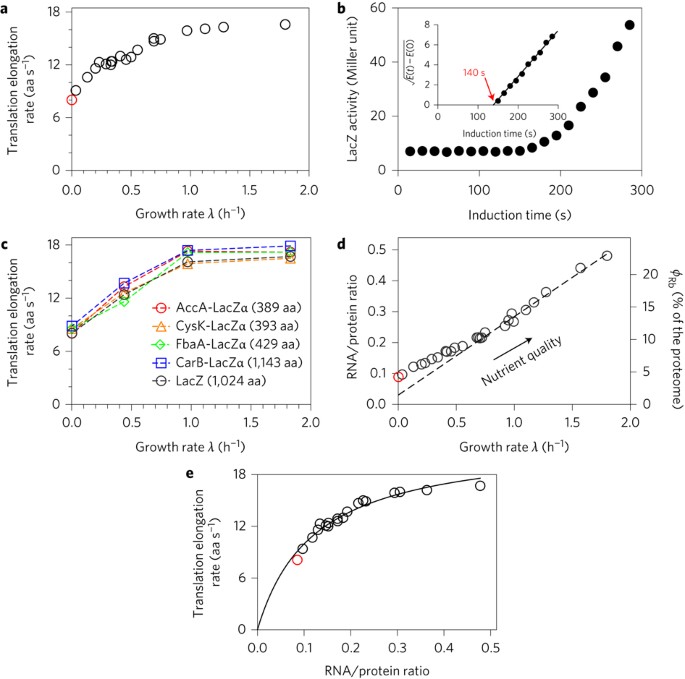
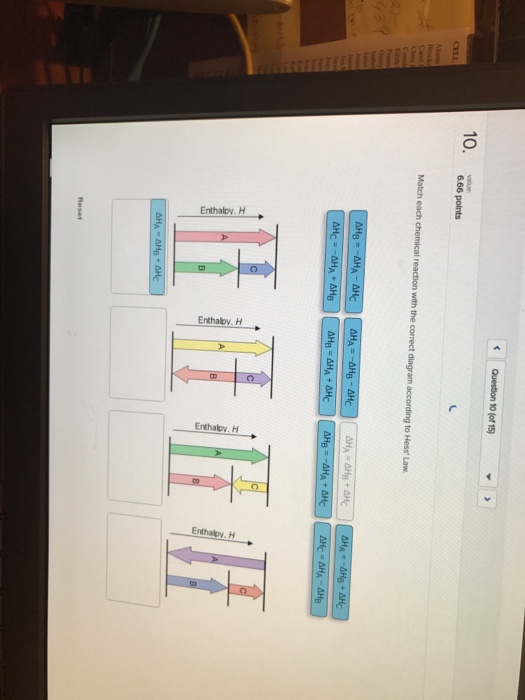
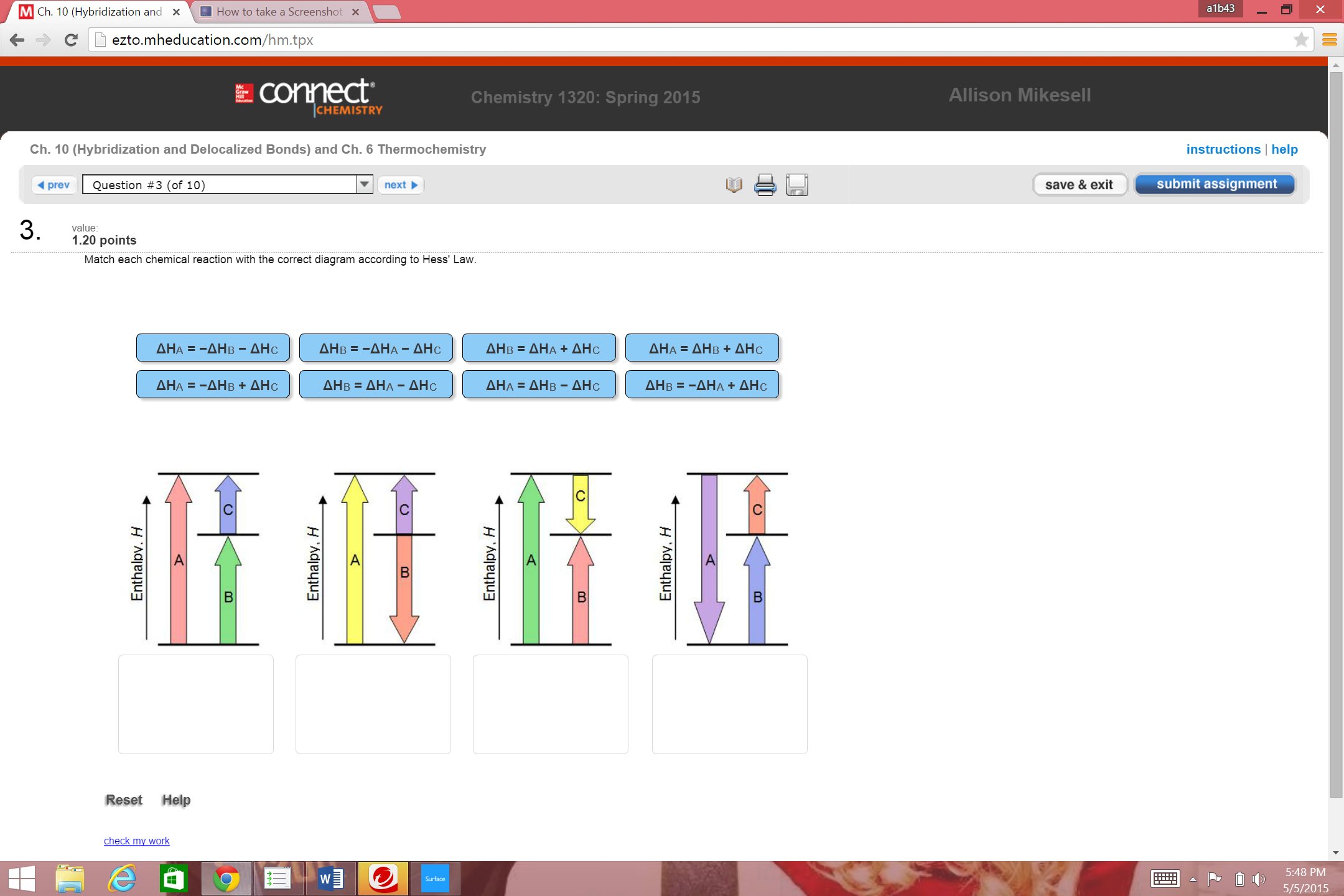
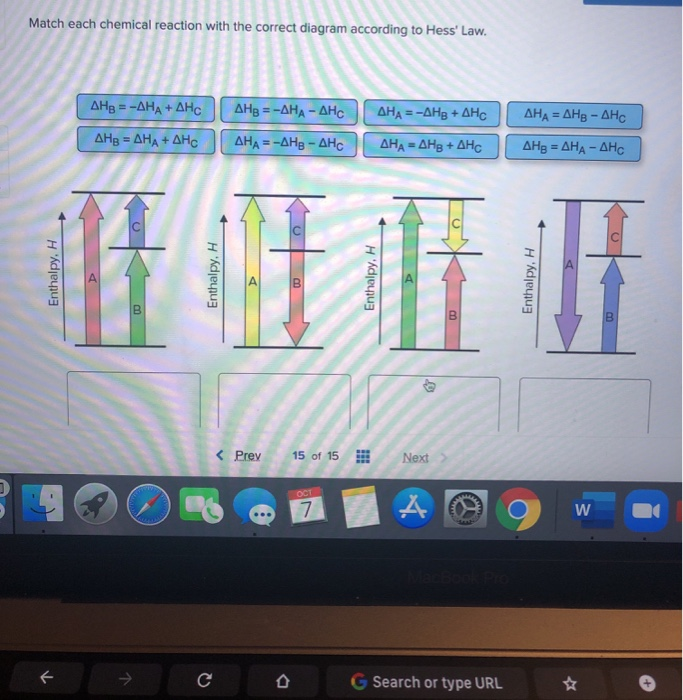
Match Each Chemical Reaction With The Correct Diagram According To Hess Law
Now that we understand that chemical is there a way around this?
Match each chemical reaction with the correct diagram according to hess law. A student failed to fill the cuvette 2/3 full with the reaction mixture. Using hess's law and standard heats of formation to determine the enthalpy change for reactions. We can judge whether the chemical reaction between the reagents was made or not according to. Equals the difference of enthalpy changes for the individual steps in the reaction.
• using hess's law, any enthalpy of reaction may be calculated using enthalpies of formation for all the substances in the reaction of interest, without knowing anything 3. It is useful to find out heats of extremely slow reaction. Hess's law states that the change in enthalpy accompanying a chemical reaction is. Agree or disagree with the statements.
So chemistry is the study of the composition and properties of matter, their changes, the conditions under which such changes take place, and the energy changes which accompany them. Calorimetry all chemical reactions involve an exchange of heat energy; According to the hess's law of constant heat summation, the total amount of heat evolved or absorbed in a reaction is all chemical reactions that take place around us might not be using heat energy always for there completion but there are. Hess's law states that the enthalpy change for a chemical reaction is independent of the route taken.
- 2006 Peterbilt 379 Fuse Box Diagram
- 2002 Chevy S10 Fuel Line Diagram
- Yanmar 3 Cylinder Diesel Engine Injector Pump
A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. According to the modern view of chemical reactions, bonds between atoms in the reactants must be broken, and the. Draw the overall chemical reaction as an enthalpy diagram (with the reactants on one line, and the products on the other line). The enthalpy of a given chemical reaction is constant, regardless of the reaction happening in one step or many steps.
This is shown in the diagram of enthalpy cycles below. Types of chemical reactions with examples. Transcribed image text from this question. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms.
A condition that determines one's 5. Cannot be determined from the enthalpy. Hess's law allows the combination of experimentally measured enthalpy changes to calculate the desired enthalpy change. Yes you are thinking about this correct.
Substances are either chemical elements or compounds. It comes from the understanding that chemical equations can be treated like algebraic equations, with the arrow acting. Match english and russian equivalents. … the reaction arrow connecting these boxes is labeled with the heat of this reaction.
In hess' law, enthalpy is independent of the mechanism of the reaction. A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. The student of law is concerned with the questions of relationships between individual citizens and the state laws and principles according to which a country is governed; This is shown in the diagram below.
Hess' law describes the conservation of energy. Equals the enthalpy change of the first step in the reaction. Caitlin bettenay 31st january 2017 law: This page explains hess's law, and introduces simple enthalpy change calculations.
The enthalpy change accompanying a chemical change is independent of the route by which the chemical change. All these questions can be answered by the branch of chemistry, which deals with the study of reaction rates and their mechanisms, called chemical kinetics. This is not the usual chemteam manner of solving hess' law problems. So hess's law tells us that delta h of this reaction, the change in enthalpy of this reaction, is essentially going to be the sum of what it takes to decompose.
According to hess's law, the overall enthalpy change in a reaction a. The following experiment has been designed to determine the enthalpy for mgo(s) using law and. Hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total enthalpy change for the reaction is the sum of all changes. Match each word on the left with the correct definition on the right.
Hess's law is a version of the general law of conservation of energy i.e. Most calculations follow from it. Although we do not release energy to this scale in the experiment, the basis of releasing potential energy from matter is still investigated as well as its relation with the laws of thermodynamics. Chemistry is concerned with the nature of fire and the structure of water, it deals with colours, catalysis and crystal.
Chemistry, by its very nature, is concerned with change. Moreover, we regularly make chemical reactions. Hess's law lab reacting mg and mgo with hcl solution to determine molar enthalpy of combustion of mg. I believe the correct answer would be that the change in enthalpy can be found by adding the enthalpies of the individual thermochemical reactions of a chemical reaction.
Hess's law is useful to calculate heats of many reactions which do not take place directly. What effect does this error. The first law of thermodynamics which can be stated as energy cannot be destroyed or created but merely changed in form or distributed in different ways. One method of calculating an enthalpy change for a process involves rearranging a set of given reaction equations with known values.
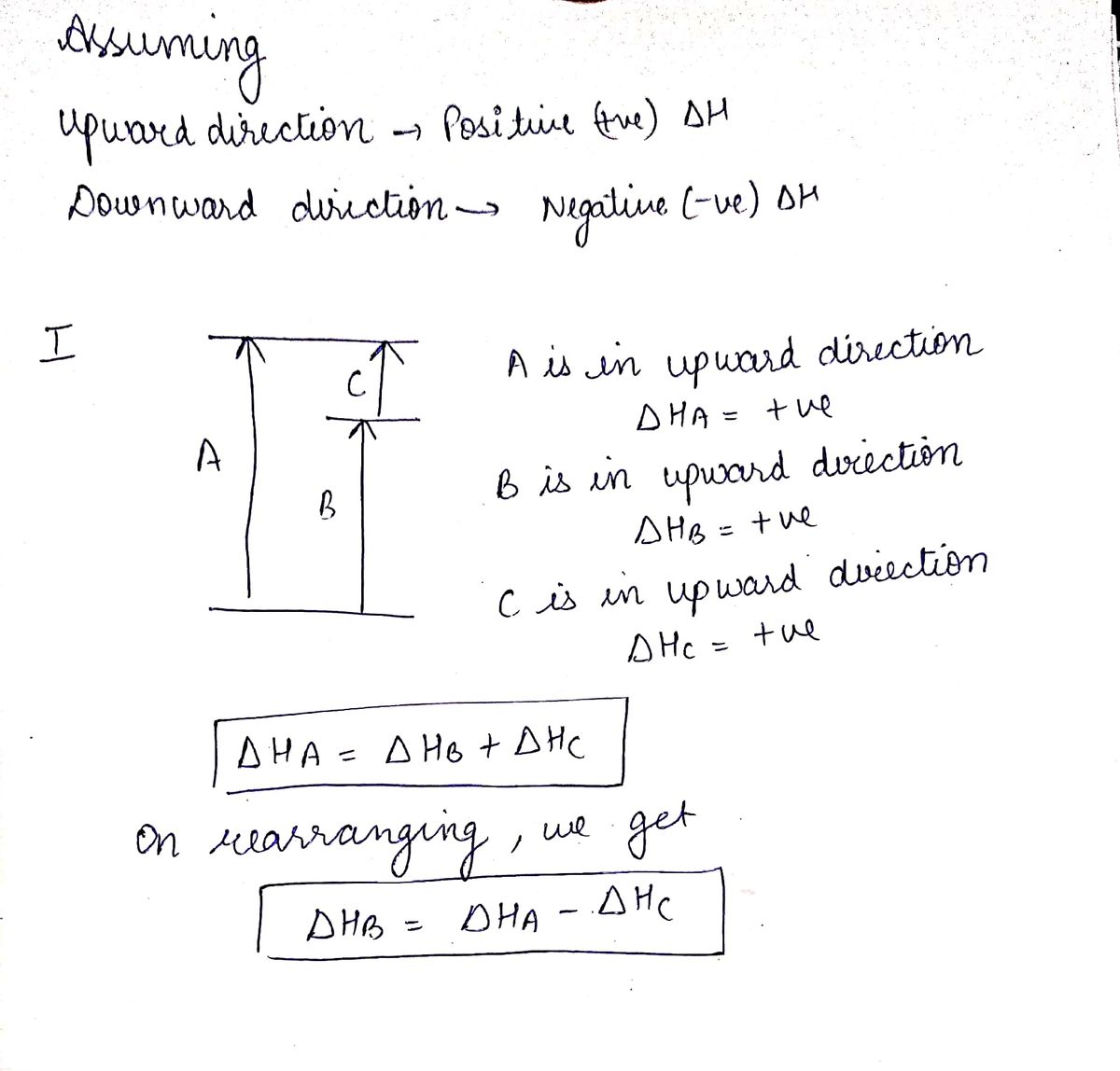
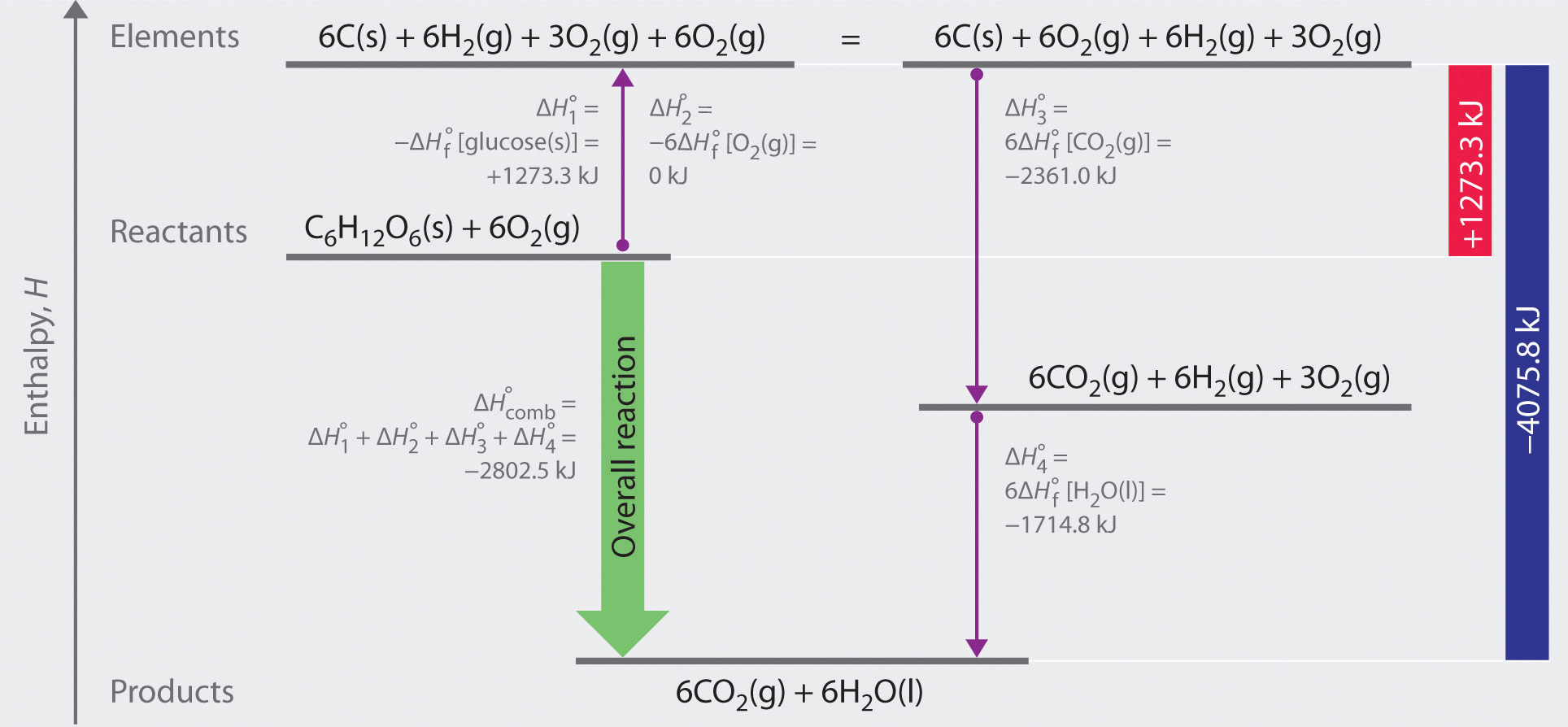
Hess's law, also known as hess's law of constant heat summation, states that the total enthalpy of a chemical reaction is the sum of the enthalpy changes for the steps of the reaction. Which is why i coped it, so as to allow you to analyze how another brain approaches these. Chemical kinetics helps us to understand how chemical reactions occur. Match each chemical reaction with the correct diagram according to hess' law.
Finding a correct path is different for each hess's law problem and may require some trial and error. Therefore, it is 0 0 0 rxn f products f reactants h n h n h n stoichiometric coefficients (moles of each reactant and for an exothermic reaction, the amount of heat evolved the by the reaction can be calculated. Using hess's law and standard heats of formation to determine the enthalpy change for reactions. Consider the following example for the formation of methane from carbon.
1) to regulate the relations a) установленная. States that, if an overall reaction takes place in several steps, its standard reaction enthalpy is the sum of the standard enthalpies of the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the dissolution of a solute. Hess's law is the most important law in this part of chemistry.