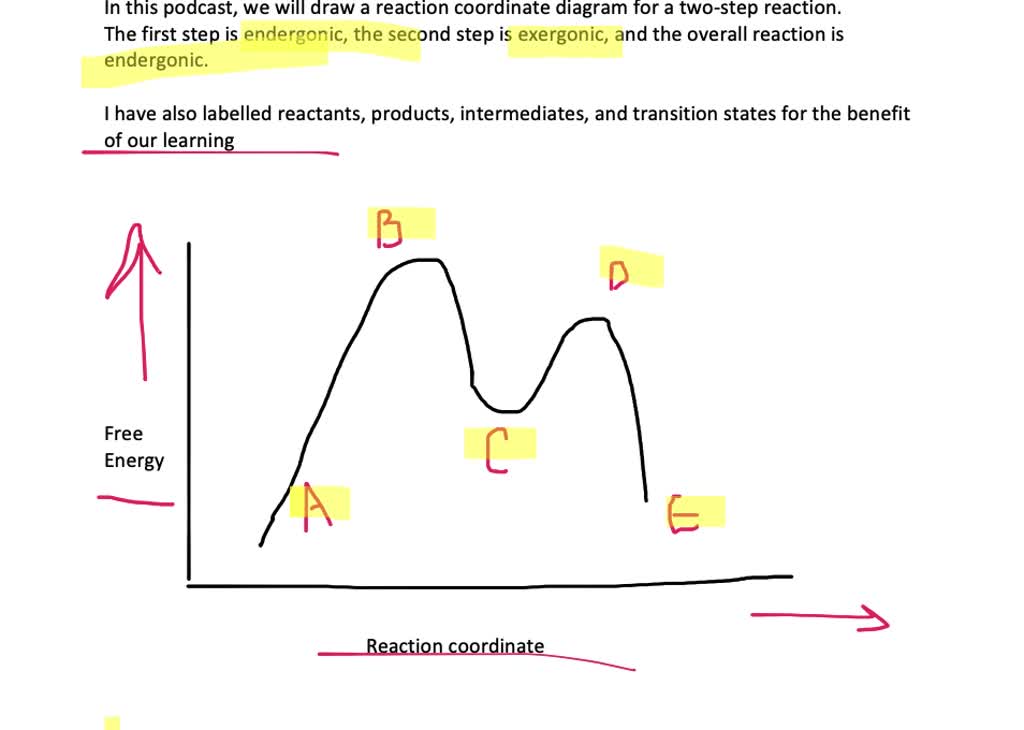
Label The Energy Diagram For A Two Step Reaction
Label the overallg, transition states, and intermediate.
Label the energy diagram for a two step reaction. Label the positions corresponding to. A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. There is a printable worksheet available for download here so you can take the quiz with pen and paper. Draw and label a pair of axes.
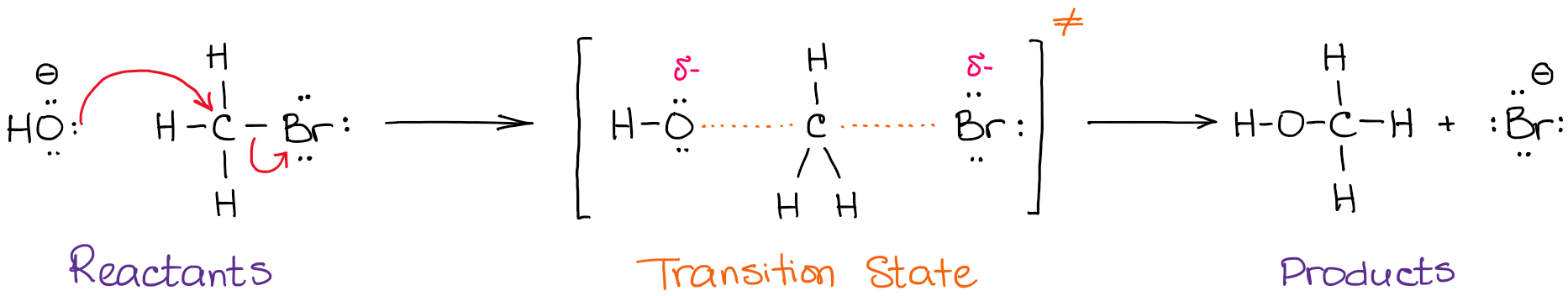
Transcribed image text from this question. Is the overall reaction so this d reacting, the media boy question be who said hey, see? The vertical axis of the diagram represents the energy of all the reactants and the horizontal axis, called reaction coordinate, represents the progress of the reaction from beginning to end. Labeling of the parts in the diagram:
State whether the transition states resemble the starting material, the intermediate, or the product. Energy diagrams are used to depict graphically the energy changes that occur during a reaction. Indicate dgrxn, as well as dg1* and dg2* for the first and second activation energies, respectively. Draw the energy level diagram.
- 2003 F250 Fuse Box Diagram Under Dash
- Simplicity Regent Belt Diagram
- 2007 Hummer H3 Stereo Wiring Diagram
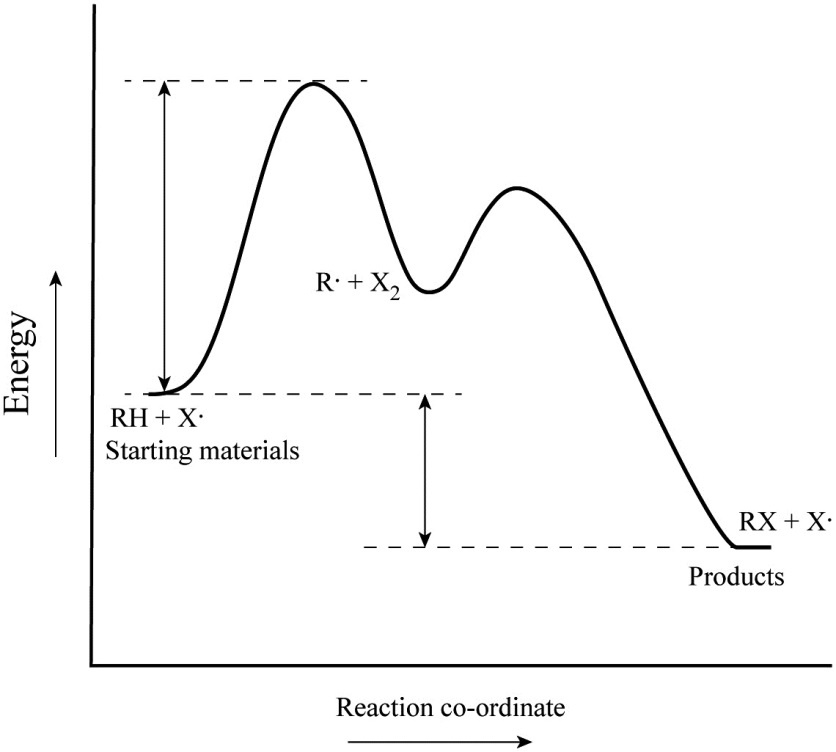
Also, two steps are involved How many intermediates are there in the reaction? The potential energy diagram can illustrate the mechanism for a reaction by showing each elementary step of the reaction with distinct activation energy (see figure below ). A potential energy diagram for an s n 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction related images label the energy diagram for a two step reaction labeling parts of coordinate youtube.
You follow a series of steps. Please check out the link below. Since 2 no2 molecules participate in the first step to form the intermediate no3, and a co molecule participates in the second, if the second step was the slow one, we would expect co to be a part of the rate law. Figure shows the energy level diagram for the reaction between methane and oxygen.
This first video takes you through all the basic parts of the pe diagram. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice consider the energy diagram for the following reaction: Shows the greatest activation energy for the forward reaction. Shows how a catalyst can affect the potential energy diagram for a reaction, and explains how a how to draw and label pe diagrams for exothermic and endothermic reactions and what effect adding a catalyst.
And then combine them in an energy diagram for the overall two step mechanism. If a reaction has h 125 kjmol and an. And now we don't be laboard out the ash and activation energy for each step. Sometimes a teacher finds it necessary to ask questions about pe diagrams that involve actual potential energy values.
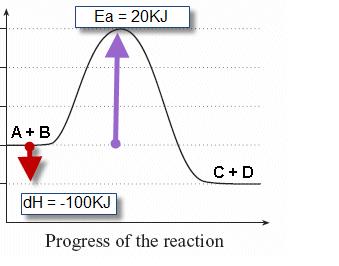
Draw and label two short horizontal lines to mark the energies of the reactants and products. For this reason, we say that it has second order kinetics. Therefore, it is an endothermic reaction. Consider the reaction energy diagram shown below.
In this process, energy is absorbed from reactant to product (i.e.δh=+ve). Energy diagrams energy diagrams depict the reaction progress versus energy. Label energy diagram 5 artatec automobil. Label the energy diagram for a two step reaction.
Watch complete videos @ www.learningchemistryonline.com organic chemistry 1. You have 1 free answer left. Activation energy is the energy required for reactant molecules to react and yield the product. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction.
5.33 an alternative course for the reaction of bromine with ethane could result in the. Endothermic reactions are defined as the reactions in which energy of the product is greater than the energy of the reactants. Energy profile diagrams for endothermic and exothermic reactions. The total energy is absorbed in the form of heat and for the reaction comes out to be positive.
A) label the electrophile and the. 1 revisit lewis acid/base reactions. Which points on the curve indicate transition states? Despite its apparent simplicity, this energy diagram conveys some very important ideas about the thermodynamics this reaction involves a collision between two molecules:
Label the reactants, transition states, reaction intermediate, activation energies, and enthalpy differences. Use the hammond postulate to explain your reasoning. Each step has its own delta h and activation energy. Which point on the graph corresponds to a reactive intermediate?
Label the vertical axis potential energy and the horizontal axis reaction coordinate. Students also viewed these (hint: Represents the reaction with the greatest change in enthaipy. Shows the lowest initial energy state for the reactants 2.
Fundamentals the starting material is the chemical substance, which is used for a chemical reaction as raw material. • the second step for reaction (a) is more exothermic than the second step for reaction (b) • the more exothermic step has a smaller ea, ea2(a) < ea2(b), and. And (a) is more likely to happen than (b).so the energetic is cl2>br2>i2. Label δh as positive or negative.