H2 Lewis Structure
The structure on the right is the lewis electron structure, or lewis structure, for h2o.
H2 lewis structure. Write the lewis structure for methane (ch4). How do you determine the lewis structure for h2c2o4? Lewis dot structures (or just lewis structures) were developed around 1920 by pioneering chemist gilbert lewis, as a way of picturing chemical bonding in molecules. Write bonds in the structure and the place remaining electrons to selected atoms in the structure to give each atom an octet.
< valence shell electron pair repulsion (vsepr) theory, along with lewis structures can be used to predict molecular geometry. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. A simple and general procedure for writing lewis structures is given in a previous post entitled lewis structures and the octet rule. Lewis structures don't tell us everything, but along with molecule geometry and polarity they are hugely informative.
Complete lewis structure by drawing atomic connectivity. Hydrogen atoms are always placed on the outside of the molecule, so carbon should. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms.
Water has the chemical formula of h2o example: It can therefore only make one bond to one other atom. Lewis structures of h2o and so2: Another straight forward lewis structure.
Two hydrogens (h) are separately covalently bonded to the central oxygen (o) atom. Find the total valence electrons for the molecule. Below is shown the lewis structure for water. The trial structure is you have eight valence electrons in your trial structure, so it has the correct number of electrons.
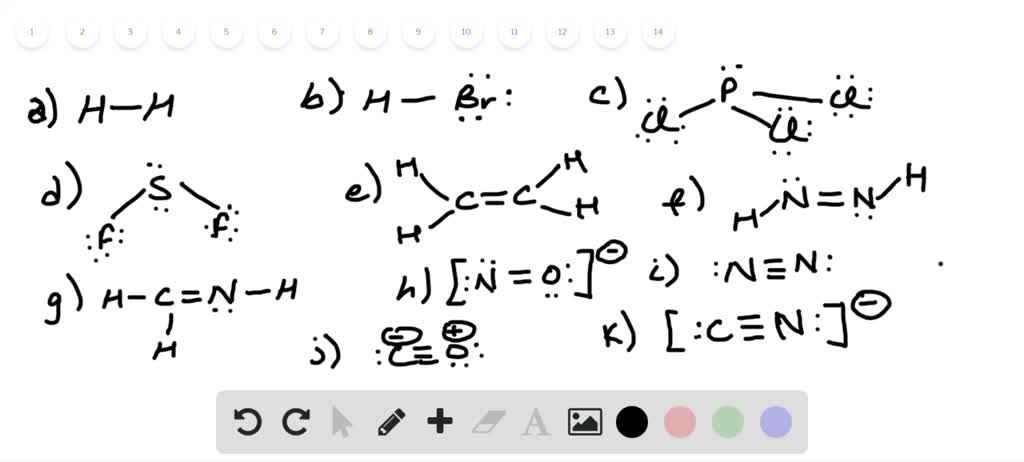
Draw the best possible lewis dot structures for each of the following compounds or ions shown below, and include resonance hybrids or isomers where appropriate: But, the lewis theory for formation of covalent bonds stands correct in almost all places, with. The bonding electrons are indicated by the dashes between the oxygen (o) and each hydrogen (h) and the other two pairs of electrons that constitute oxygens octet, are. Steps for writing lewis structures.
Remember that hydrogen only needs two electrons to have a full outer shell. Write lewis symbols for neutral atoms and ions. We start by writing symbols that contain the correct number of valence electrons for the atoms in example: It assumes that bonds and lone pairs repel each other, and will arrange themselves to be as far from each other as possible.
When we have an h (or h2) in front of a polyatomic molecule (like co3. Lewis dot structure of atoms link. Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. So, according to valence shell electron pair repulsion (vsepr) theory, all of these will spread out as far as possible, which will end up to giving us the shape of h2o.
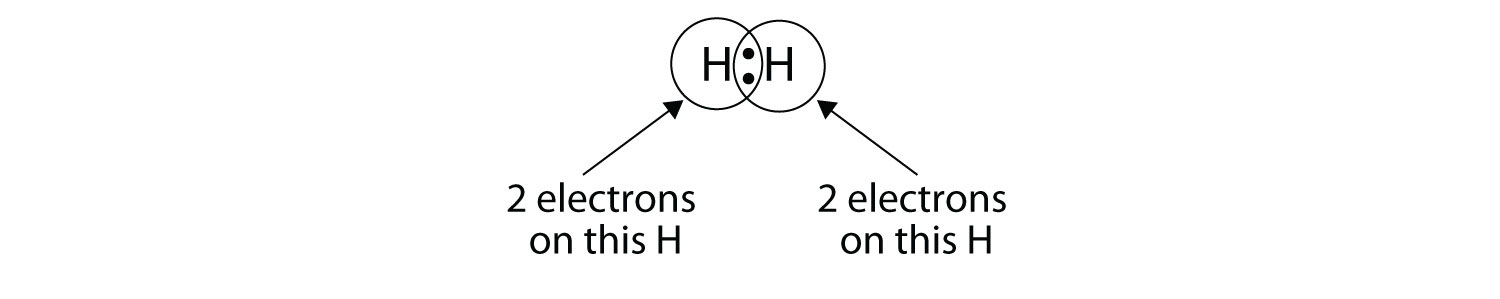
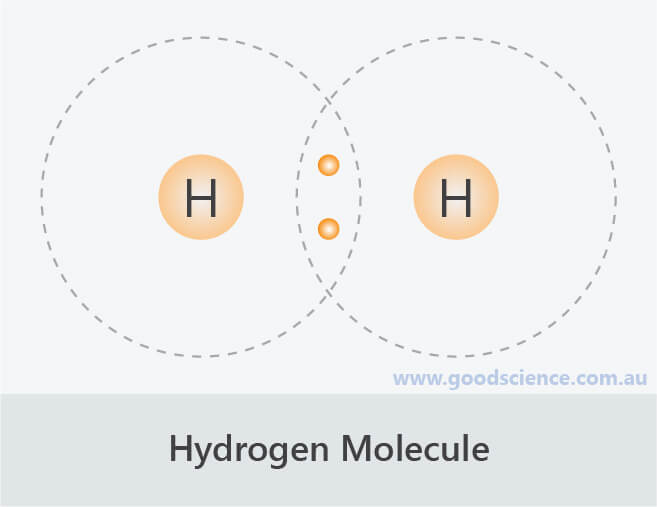
Since valence electrons are typically represented as dots, these the formula for water is h2o. For the h2 lewis structure, calculate the total number of valence. You can find a procedure for drawing lewis structures at this location. In lewis dot structures each dot represents an electron.
Most lewis structures will follow the octet rule, which states that the outer (valence) shell is stable when it has eight electrons. The diagram opposite shows the lewis structure for the water molecule, h2o dots represent electrons which are not involved in bonding. Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons.
Counting valence electrons yields eight total (six from oxygen, one each from the two hydrogens). How do i draw the lewis electron dot structure of cyanamide cn2h2? General rules for drawing lewis structures. The lewis structure of each of these atoms would be as follows:
There are also two pairs of electrons around the oxygen, which you can see at the lewis structure. One good example is the water molecule. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the. Most lewis structures you encounter will be covalent bonds.
Writing lewis structures with the octet rule. Drawing the lewis structure for h2o. The formula of acetic acid is often written as ch3co2h, because this molecule contains the following skeleton structure. Formaldehyde (h2co) is the simpiliest of a class of functional groups.
We draw lewis structures to. Discover the bonding arrangement of atoms Thus far in this chapter, we writing lewis structures with the octet rule. Ad by raging bull, llc.
It is helpful if you You have a total of 8 valence electrons available to fill the octets of oxygen and hydrogen. For more complicated molecules and molecular ions, it is helpful to follow the. You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell.
Draw lewis structures depicting the bonding in simple molecules. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping. The lewis structure of a compound can be generated by trial and error. There are many exceptions to this rule, but it should be used as a general guide for creating lewis structures.
All valence electrons of the atoms in lewis structures must be shown. Lewis structures show how the electrons are arranged in the molecule.