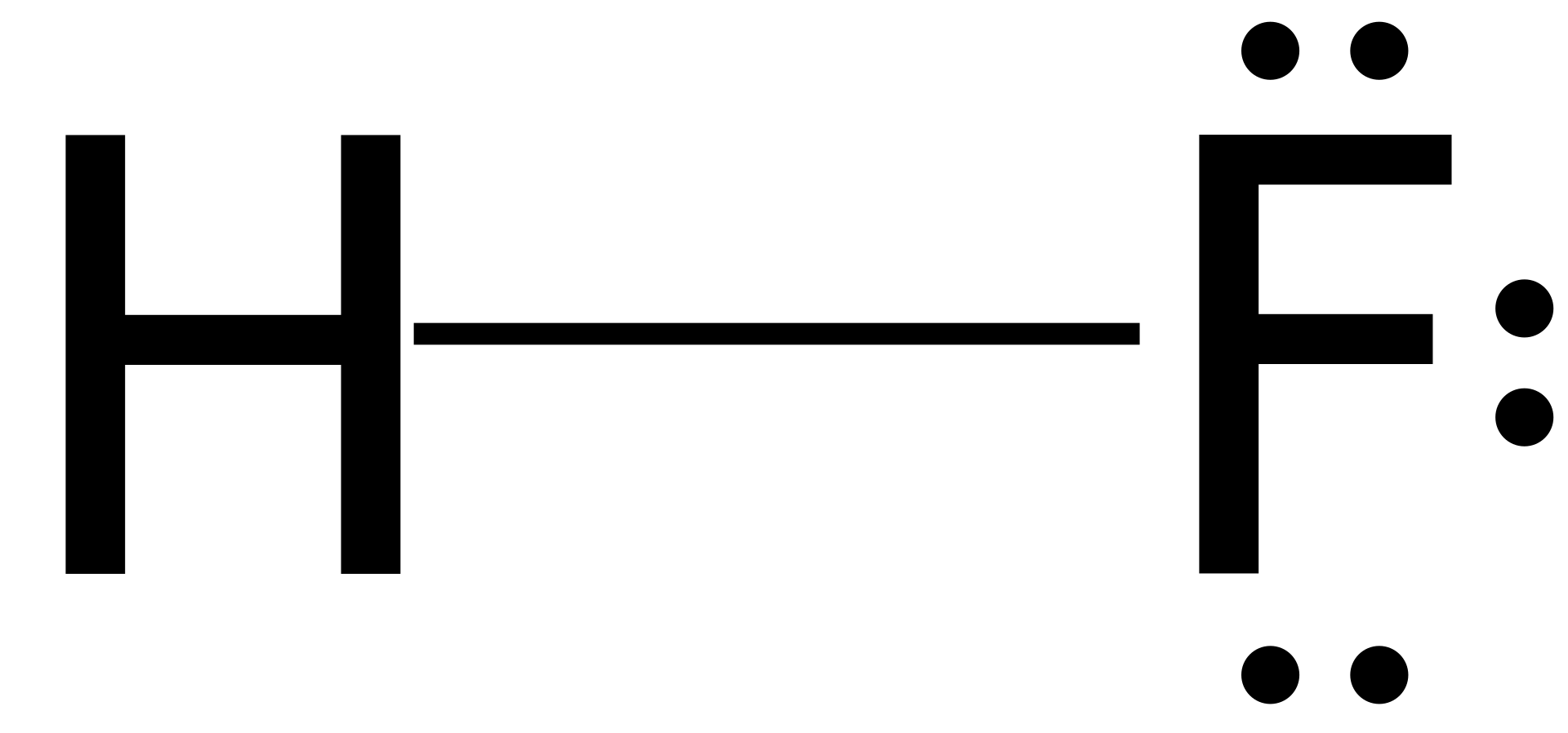Electron Dot Structure For H2
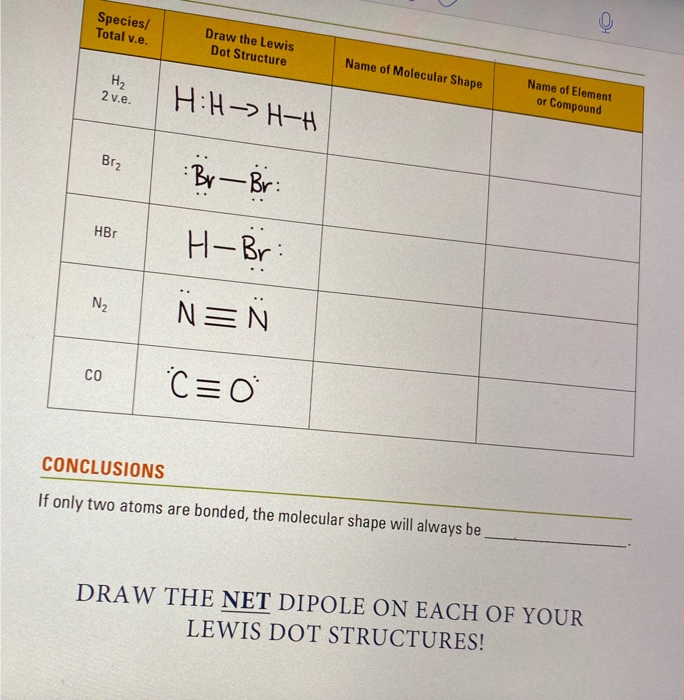
Draw the best electron dot structure for each compound listed.
Electron dot structure for h2. This allows us to track the electrons better. This is a structural formula, making the electron dot structure easy to draw. This is called a single covalent bond , when two atoms are. Write electron dot structures for each of the following elements.
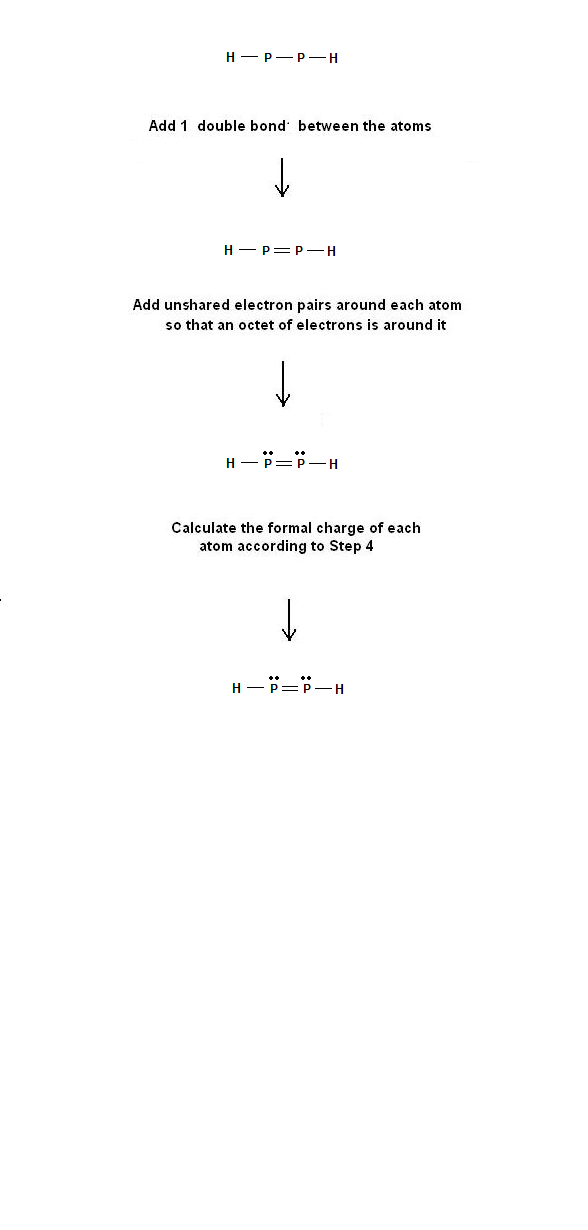
Electron dot structure for ccl2h2. How do i draw the lewis electron dot structure of cyanamide cn2h2? H2 o⟶ (refer to image 2). Electron dot structure for h2 is • h•.
So to complete the valence, each. Let's produce a lewis dot structure for: In order to eject an electron from a metal, a photon of a certain minimum energy must strike the sur. For h2 in a minimal basis, the simplest choice for hˆeff.
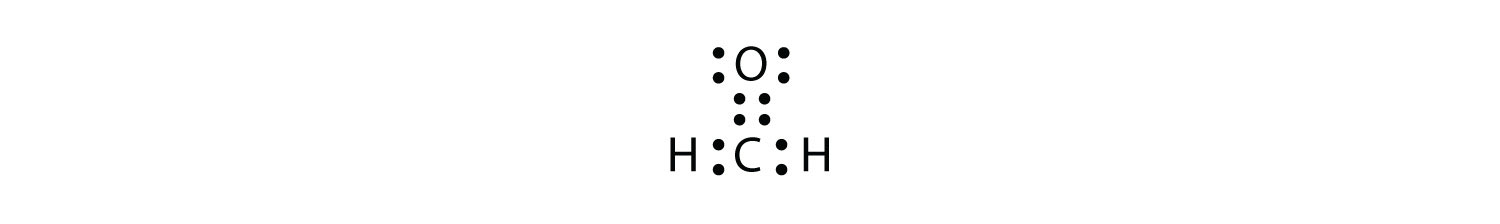
First you calculate the number of electrons required to give everybody a noble gas configuration that would be 2*8 =16 for the o atoms and 2*2 = 4 for the h atoms. The structure on the right is the lewis electron structure, or lewis structure, for h2o. This c atom is double bonded to another c atom. Dot structures show what's in the outermost shell (the valence electrons).
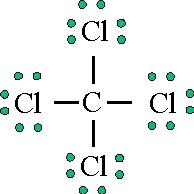
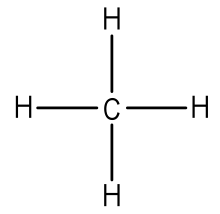
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Diagrams that show valence of electrons as dots. Lewis dot structures can be produced by following a sequence of steps. Organizing information complete the table below.
On the right is an h 2 molecule showing the electron cloud overlap. How to draw double and triple bonds using dots to represent valence electrons. Always make sure that the completed dot structure has the same number of individual valence electrons you started. Electron dot structures, also called lewis structures, give a representation of the valence electrons surrounding an atom.
A simple and general procedure for writing lewis structures is given in a previous post entitled lewis structures and the octet rule. Lewis structures, also known as electron dot structures, are named after gilbert n. The electron dot structure are as follows Electronic structure theory of real molecules.
His appropriately, and we see that we have two electrons accounted for in each oxygen. First, to construct the lewis dot structure for age to go to, and second, to compare information about that dot structure to a table 18.2, according to it's one valence electron. You can draw a lewis dot structure for any. We begin from the left, with two h atoms attached to a c atom.
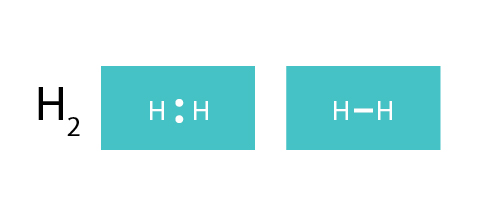
Where it should be remembered that within the bo approximation, rab is just a number. The shared pair of electrons is shown as two dots in between the two h symbols (h:h). Prentice hall biology assessment answers unit 10 (291 reads) nuclear reaction webquest. In all cases, except h2o, the.
Sometimes we use an x instead of a dot to represent an electron. Electron dot structure also called lewis dot structure arranging the dots… dots are placed around a symbol to indicate the number of valence ehow each hydrogen atom wants two electrons, and they already have two electrons… no dots need to be added the kelter method…h2o account for. After determining how many valence electrons there are in h2, place them around the central atom to fill their the outer shells. The number of ions of opposite charge that surround each ion in a crystal.
Thus making a electron dot structure as per below. Electron dot structure for csf2. Relation between energy and wavelength is given as: With two bonding pairs and two lone pairs, the oxygen atom has the unpaired electron is usually placed in the lewis dot structure so that each element in the structure will have the lowest formal charge possible.
Electron dot diagram for ch3oh. How can you determine the lewis dot structure of h2o2? Very conveniently, the group number corresponds to the number of count the total valence electrons: Each valence electron is represented by one dot, thus, a lone atom of hydrogen would be drawn as an h with one dot.
Lewis, who described them in a 1916 article titled, the atom lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. Approach requires one to define an effective one electron hamiltonian, hˆeff.