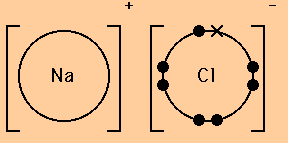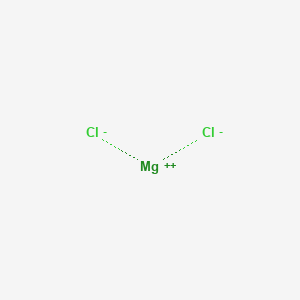Dot And Cross Diagram Of Magnesium Chloride
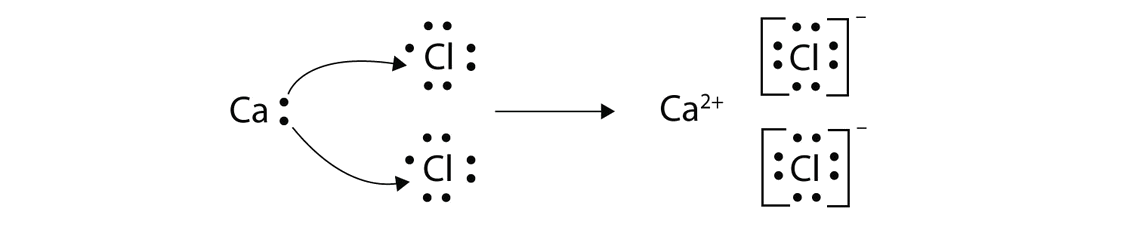
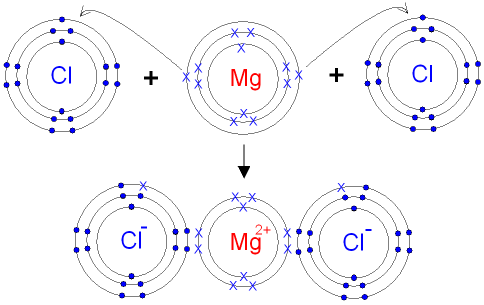
Magnesium reacts with chlorine by losing two electrons and on the other hand chlorine reacts by gaining the electrons lost by magnesium.
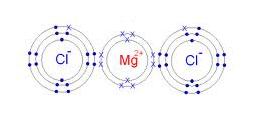
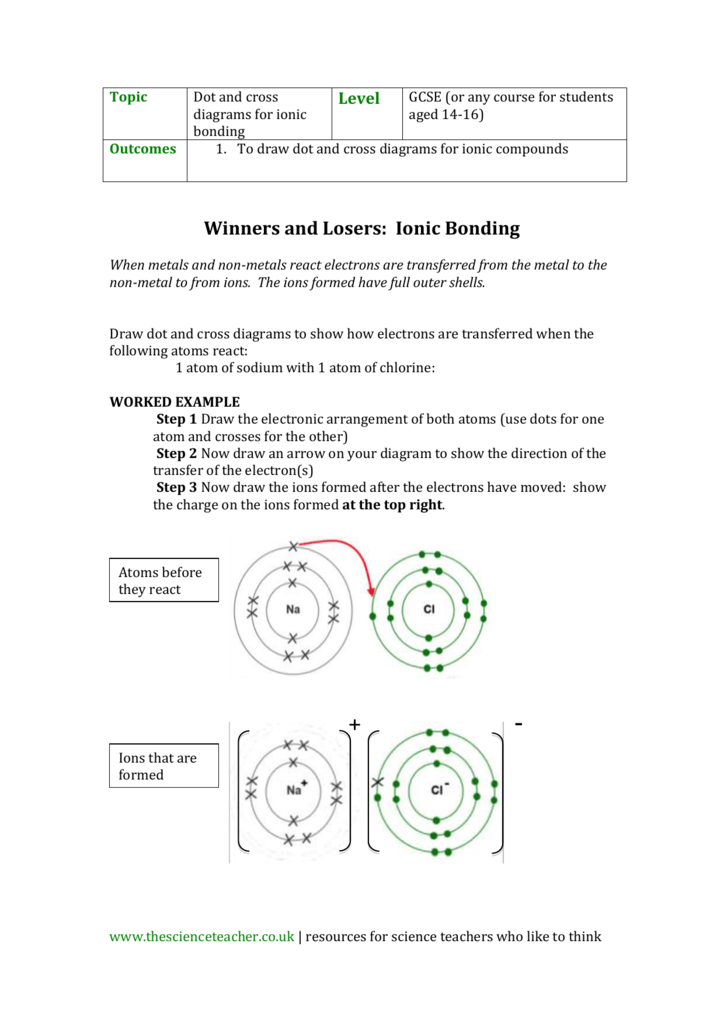
Dot and cross diagram of magnesium chloride. Shows the ionic bonds between ions by the loss and gain of electrons. Diagram to represent any giant metallic structure. A write an equation for the reaction, including state symbols. (i) draw a dot and cross diagram to show the electronic structure of the compound magnesium chloride (only the outer electrons need be shown).
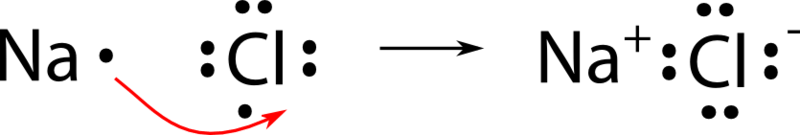
When drawing dot and cross diagrams of ionic compounds the ions must not touch each other; Chloride ions each have a single negative charge. So the diagram shows the sodium atom losing the only electron in its outer shell, the chlorine atom adding one electron to the seven it already has, and the charges of the two ions formed with sodium having an empty outer shell and the chloride. Draw a dot and cross diagram to show the bonding in hydrogen chloride and label a lone pair of electrons.
(b) draw a dot and cross diagram for a molecule of hydrogen chloride. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. 3 magnesium reacts with bromine to form magnesium bromide. (b) draw a dot and cross diagram to show the arrangement of outer shell electrons in one molecule of water.
- How A Livewell Works Diagram
- 2008 Dodge Ram Headlight Wiring Diagram
- Honda Fourtrax 300 Carburetor Diagram
I show you where magnesium is on the periodic table and how to determine how many valence electrons magnesium has. Magnesium fluoride is an inorganic compound with the formula mgf2. Magnesium chloride is the name for the chemical compound with the formula mgcl2 and its various hydrates mgcl2(h2o)x. Anhydrous mgcl2 contains 25.5% elemental magnesium by mass.
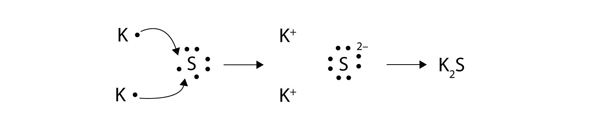
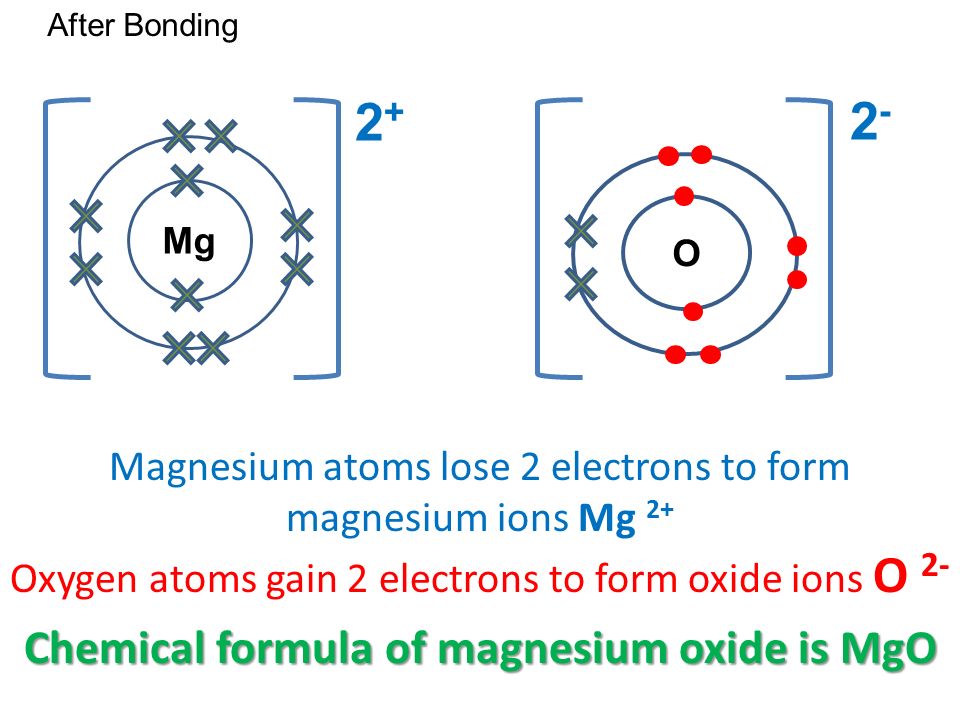
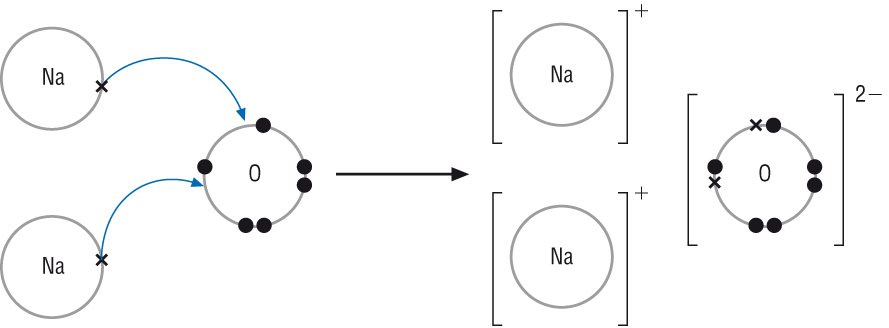
When magnesium reacts with sulfur to form magnesium sulfide the electronic configuration of … a. Hence the use of beryllium chloride (2 bonding pairs only). Structure and bonding in sodium chloride. Draw 'dot and cross' digrams to show the formation of :
Draw electron dot representation for the formation of sodium chloride. Magnesium chloride and sodium chloride these are the salts used in a new saltwater pool chlorinating system but even without the chlorinator its good for you and makes the pool feel like a mineral bath. The beryllium atom can have only four electrons in its outer shell and still be energetically stable. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride.
Chloride ion dot structure diagram labeled michaelhannan co. Diatomic molecules, including hydrogen, oxygen, nitrogen. Calcium oxide magnesium chloride 2 10 chemical formulae of ionic compounds write the formula of magnesium carbonate. Sodium chloride, nacl magnesium chloride, mgcl2 potassium oxide, k2o calcium oxide, cao aluminium oxide, al2o3 magnesium nitride, mg3n2.
For the charges to cancel out in the neutral salt magnesium chloride, they must be in a ration of 1:2. 2 ionic bonding hence the 4 more practice 1. In the image below i have provided the dot and cross diagram for. These salts are typical ionic halides, being highly soluble in water.
Determine the type of bonding. Magnesium changes from 2,8,2 to 2,8 and an mg+ ion is formed. The bonding agent is the basic salt of magnesium chloride. What does the lewis dot diagram of magnesium look like?
Your diagrams should only show outer shell electrons. The hydrated magnesium chloride can be extracted from brine or sea water. (i) ionic, as in sodium chloride, magnesium oxide (ii). So the formula of magnesium chloride is mgcl2.
(a) the dot and cross diagrams show how a sodium atom bonds with a chlorine atom to form sodium chloride. The water is also good for the. Determine the formula of the ionic compound, using the cross method. These salts are typical ionic halides, being highly soluble in water.
How to draw the dot and cross diagram for magnesium chloride. Select the dot and cross diagram which correctly represents the bonding in water (outer shells only) 8. Magnesium chloride is the name for the chemical compound with the formula mgcl2 and its various hydrates mgcl2(h2o)x. In this a reaction takes place and thus a compound known as magnesium chloride is formed.
*(iii) suggest why the melting temperature of magnesium oxide is higher than that of magnesium chloride, even though both are almost 100. Ignite a ribbon of magnesium, and introduce it in the jar of carbon dioxide. (a) magnesium bromide is an ionic compound. 12 show by using dot and cross diagram the bonding in these ionic substances.
Gcse chemistry the reaction between magnesium and chlorine. Predict, with reasons, the bond angle in an ammonia. Antibody a solution in 0 1 acetic acid containing 50 mm magnesium. 3 magnesium chloride can be made by reacting solid magnesium carbonate, mgco3, with dilute hydrochloric acid.
Dot and cross diagram for magnesium oxide. (ii) using dot and cross diagrams explain how magnesium chloride is formed from atoms of magnesium and chlorine.