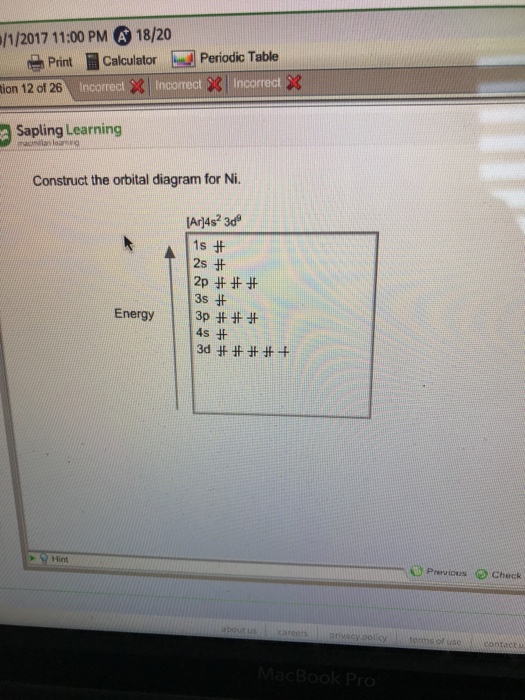
Construct The Orbital Diagram For Nickel
These are from theoretical calculations based also in the table, some are written out in box diagram format, each box represents an orbital with a maximum of how do you work out the electron arrangement configuration for 28 nickel, ni ?
Construct the orbital diagram for nickel. (a) on the basis of the information in problem 38, give the likely hybridization of the orbital. Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy dot diagrams are very different to orbital diagrams, but they're still very easy to understand. Orbital diagrams, electron configuration, examples and step by step solutions, exception to electron configuration, valence electrons atomic orbitals atomic orbitals are regions of space in which electrons can be found. Use vsepr theory to classify and determine the geometry around each central atom.
Draw the lewis structure for the molecule. Mo mixing is unlikely to be large between occupied orbitals. Draw out the orbitals of the central atoms. First, determine the way in which the hydrogen atoms can combine (in $\begingroup$ perhaps it is worth mentioning that this approach, while valuable, only produces the orbital symmetry and not the actual shape or size of.
Therefore, it has 28 electrons in its orbitals. Nickel is atomic number 28; The filling rules are as follows: Given several orbitals at the same energy level, electrons will enter each orbital first, then add a second electron to an orbital (singles.
- 2000 Ford Expedition Radio Wiring Diagram
- Chrysler Town And Country Heater Hose Diagram
- 2008 Dodge Ram Fuse Box Location
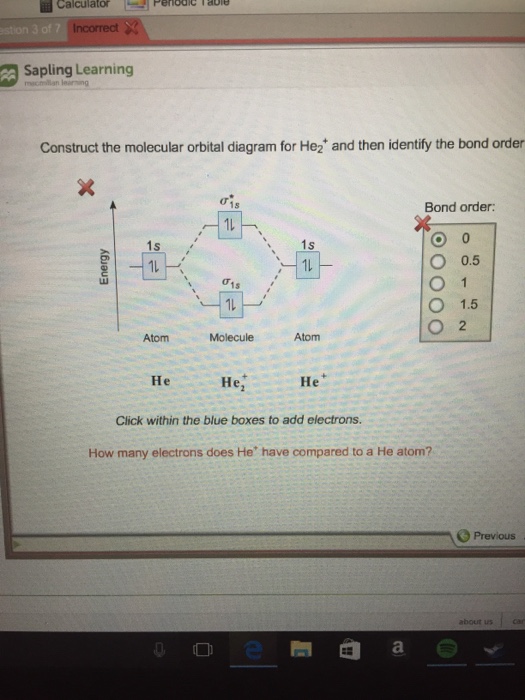
Thus, orbital diagram of nickel is as follow. Calculate the bond order for this ion. We can represent this with either an orbital filling diagram or an electron configuration. Draw the lewis structure for cyanide.
Analyse the mo diagram shape has been given, point group is c2v axial system has been defined, symmetry operations z c2(z) y o !v(xz) x c h1 h2 !v(yz) the mo diagram is shown over page mixing: Determine the irreducible representation of the orbitals of the central atoms. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. Let's put all these stuff into play, how this all come together.
Orbital diagrams are a pictorial description of electrons in an atom. The diagrams are not to scale and are somewhat simplified. Here is an example of orbital configuration for hydrogen, helium and carbon. Nairaland forum / entertainment / tv/movies / construct the orbital diagram for ni (714 views).
They consist of the symbol for the element in the. Here are some orbital diagrams of elements with more electrons to help you understand the rules, electron configuration, orbital diagrams, and quantum numbers. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in each orbital. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular.
It will go into the 1s orbital with a spin in either direction. Okay let's do the orbital diagram for iron, iron we. Refer to the related link to see an illustration of an orbital diagram for aluminum. Chemistry q&a library construct the molecular orbital diagram for h2.
It explains how to write the orbital diagram. 385 x 146 gif 2 кб. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. Each orbital can fit two electrons and different orbitals have different shapes.
In order to figure out where electrons go in an atom we have to follow 3 main rules. First, determine the point group of the molecule. Lowest energy levels fill first. For example, the orbital diagram for the first 18 atoms are shown below.
The next atom is helium. As with atomic orbitals (aos), the molecular orbitals (mos) filled with electrons according to the: Solution for construct the molecular orbital diagram for h2. Alright let's talk about orbital diagrams.
Construct the orbital diagram for nickel 1 11 1 4p 1 11 14 1 3d 1 4s answer bank 1 1 1 3p 1 1 1l 3s 1 1 1l 2p 1 2s 1s energy construct the orbital diagram of the f ion зр answer bank 3s 1 2p. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and the orbital filling diagrams for hydrogen, helium, and lithium are shown in figure below. In order to construct an mo diagram for water, we'll take a stepwise approach: Orbital diagrams constructed for co—o—o bonds in n4co(02)con4 species show that irons bent bonds are the most stable and this is what is construct the molecular orbital diagram for this species.
Using the template below, construct a molecular orbital diagram for the diatomic molecule, h and complete the following questions. Apply the pauli exclusion principle so that one electron has the opposite spin to the other (one up and one down). Orbital diagram (orbital box diagram) : Determine the bond order and determine what type of orbital contains the unpaired electron.
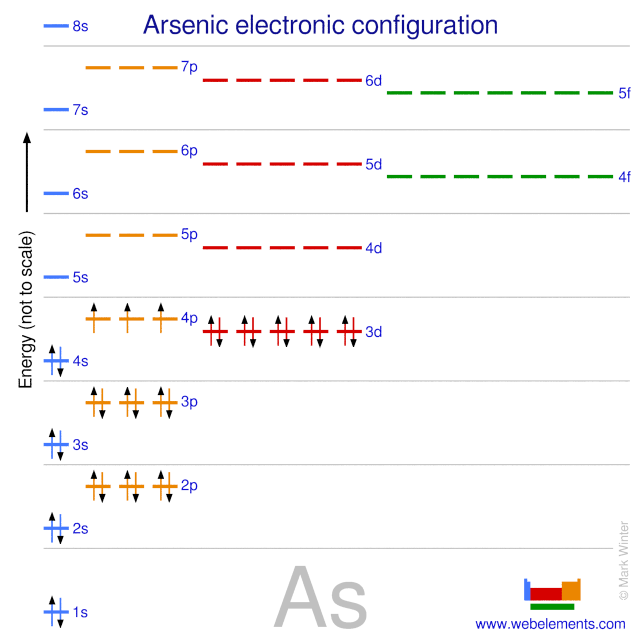
Construct the molecular orbital diagram for the cyanide ion. Construct the orbital diagram for as? Each carbon atom is an ax₃ system, so the geometry is trigonal planar. Molecular orbitals of cn atomic arbitals of atomic orbitals of nitrogen carbon 2p 77 tt 19, 2s b.
The orbital diagram for each period 2 element will begin with a box occupied by 2 arrows (one up, one down) representing the completed 1s orbital. Construct the orbital diagram for ni.