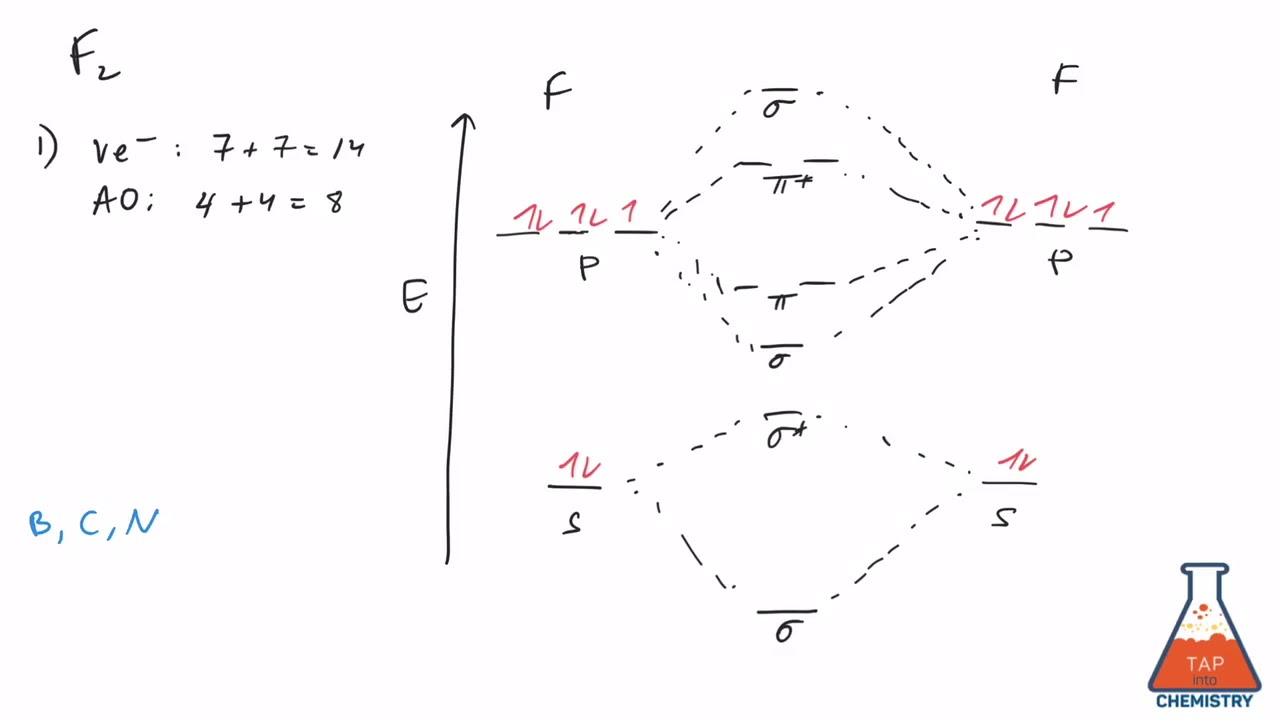
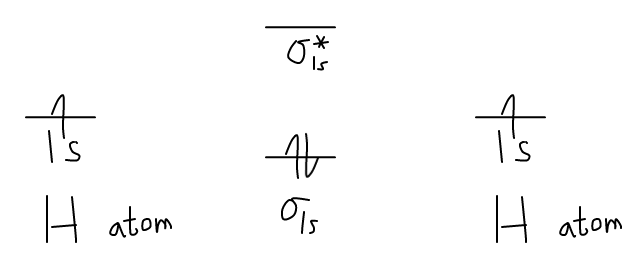Construct The Molecular Orbital Diagram For H 2
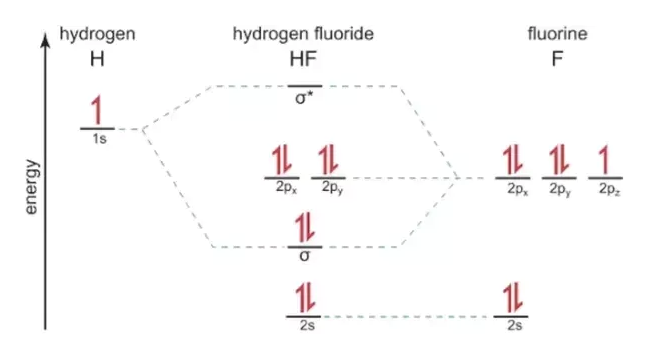
Analyse the mo diagram shape has been given, point group is c2v axial system has been defined, symmetry operations z c2(z) y o !v(xz) x c h1 h2 !v(yz) the mo diagram is shown over page mixing:
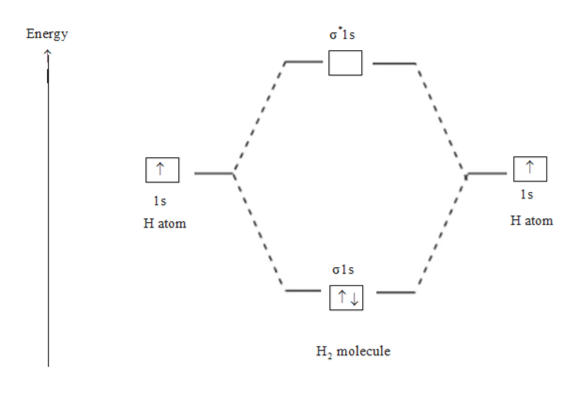
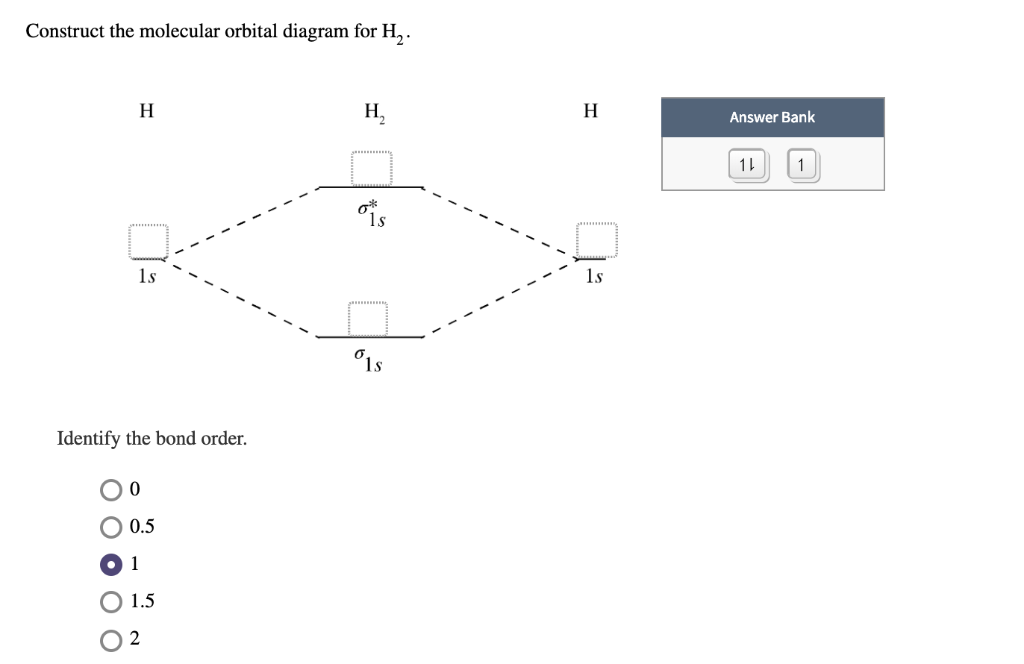
Construct the molecular orbital diagram for h 2. Mo mixing is unlikely to be large between occupied orbitals. When constructing the molecular orbital diagram, the atomic orbitals for each atom involved in the bond are drawn on the outside edges of the diagram; Neon atom has 10 electrons and its electronic configuration is. Since 1s shell of bonding orbital can accommodate only two electrons.
Unfortunately this equation does not properly account for temperature dependence of reaction rates. Write the ground state molecular orbital electron contiguration | molecule enetsy level diagram including each atom's energy. As the bond order value for molecule is zero, it is unstable and cannot exist. The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most construct a molecular orbital diagram of the kind shown in this lesson for a simple diatomic molecule, and indicate.
Bonding order is 1, and it is diamagnetic. Use these to generate a sigma bonding and a sigma antibonding molecular orbital. The molecular orbital diagram for b₂ then becomes. What is the bond order of co quora.
- John Deere Lx176 Belt Diagram
- 2003 Toyota Sequoia Jbl Radio Wiring Diagram
- How To Adjust Vertical Float Switch Sump Pump
The first major step is understanding the difference between two major theories if you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it. Construct the molecular orbital diagram for h2 and then identify the bond order. One electron in bonding orbital; Draw the molecular orbital energy level diagram for the following substances, and complete the tables.
Given,the mass of ch3oh = 14.6 gand the mass of h2o = 184 gwe know that, the molar mass for ch3oh =. Transcribed image text from this question. Construct two molecular orbital diagrams, 1 for the bonding of c and b, and the other for n and f. The mos go between what type of bond would you expect it to have?
The average energy of the molecular orbitals must be the same as the average. Molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms, that is, in terms for example, to give you a glimpse at where we are headed, the following are orbital diagrams for o2 and o. It's very confusing for me please help. First, determine the point group of the molecule.
The collision frequency, between a and b molecules, is , based on the kinetic model of a gas. Apply molecular orbital theory to determine the bond order in na2. Determine the irreducible representation of the orbitals of the central atoms. The molecular orbital energy diagram predicts that h2 will be a stable molecule with lower energy than the separated atoms.
If the phase changes the bond becomes a pi bond π bond. Molecular orbital diagram for hydrogen gas (h2). Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. For both please list the bond order and magnetic properties of themolecule.i really don't understand how to do molecular orbital diagrams.
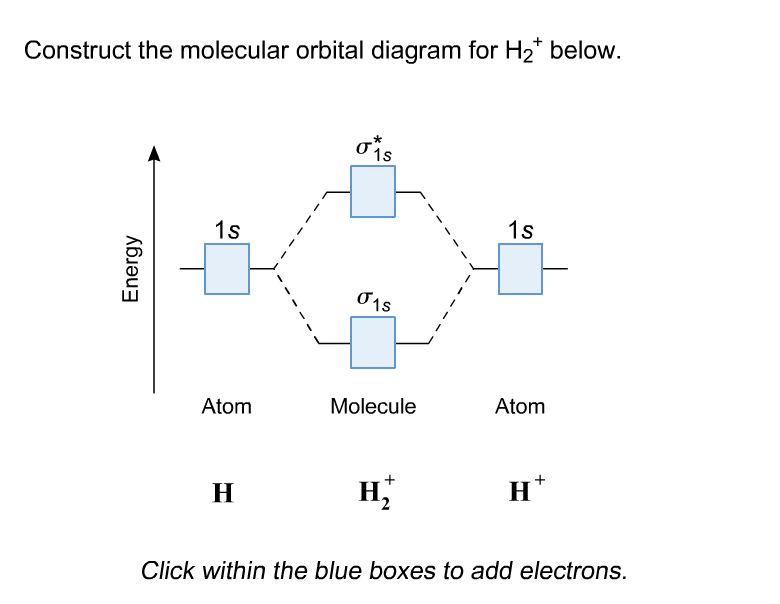
We use the pauli exclusion principle and hund's rule to fill the orbitals in an aufbau process. Use the drawing of mo energy diagram to predict. Click within the blue boxes to add electrons. Last time you learned how to construct molecule orbital diagrams for simple molecules based on the symmetry of the atomic orbitals.
Draw out the possible bonding interaction of the orbitals from the peripheral atoms. The molecular orbital diagram of hypothetical molecule is given in the attachment. Let me explain the molecular orbital diagram of n2 using its diagram. We will consider the molecular orbitals in molecules composed of two identical atoms (h2 or cl2, for example).
(d) this plot of the square of the wave function (ψ 2 ) for the adding two atomic orbitals corresponds to constructive interference between two waves, thus reinforcing their intensity; A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Discussed in this video are. Draw out the orbitals of the central atoms.
C) when two electrons occupy the bonding molecular orbital above, what type of bond results? One atom of nitrogen has 7 electrons so a n2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bon. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Each line in the molecular orbital.
Fill from the bottom up, with 2 electrons total. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Construct a molecular orbital diagram for he2 and calculate its bond order. How does bond order correspond to phase?